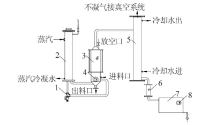
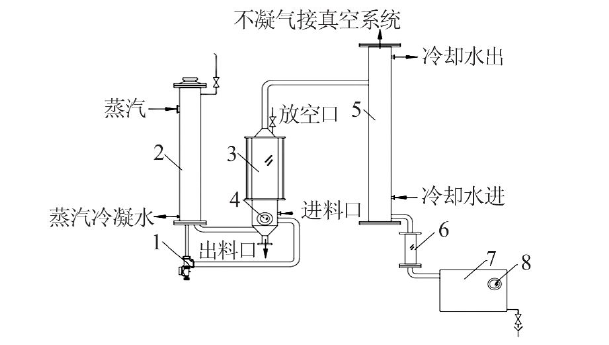
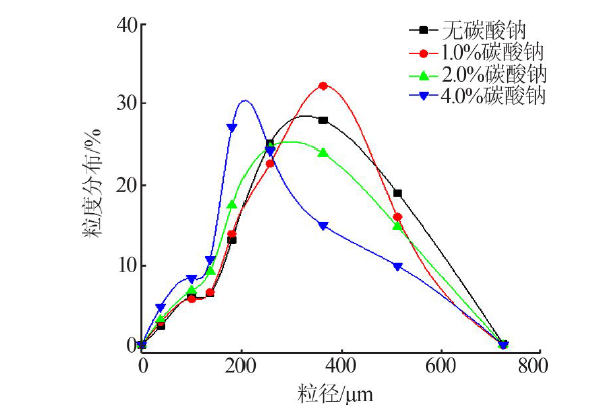
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (4): 123-127.doi: 10.19964/j.issn.1006-4990.2021-0397
• Industrial Techniques • Previous Articles Next Articles
Effect of impurity in alkaline washing oxidation process on crystal size of sodium sulfate
LIU Zhuang1( ),AN Jimin2,LI Yongjun1,CHEN Xing1,ZHAO Yigang1,ZHAI Ruiguo2(
),AN Jimin2,LI Yongjun1,CHEN Xing1,ZHAO Yigang1,ZHAI Ruiguo2( )
)
- 1. PetroChina Changqing Oilfield Company,Jingbian 718500,China
2. Tianjin Zhongtian Chem.Eng.Co.,Ltd.
-
Received:2021-07-02Online:2022-04-10Published:2022-04-18 -
Contact:ZHAI Ruiguo E-mail:495465929@qq.com;zrg-wait1986@163.com
CLC Number:
Cite this article
LIU Zhuang,AN Jimin,LI Yongjun,CHEN Xing,ZHAO Yigang,ZHAI Ruiguo. Effect of impurity in alkaline washing oxidation process on crystal size of sodium sulfate[J]. Inorganic Chemicals Industry, 2022, 54(4): 123-127.
share this article
Table 1
Technical index requirements for anhydrous Na2SO4 products and industrial anhydrous Na2SO4(GB/T 6009—2014)"
| 项目 | w (Na2SO4)/ % | w (水不溶 物)/% | w [钙和镁 (以Mg计)]/ % | w [氯化 物(以Cl 计)]/% | w [铁 (Fe)]/% | w (水分)/ % | 白度 (R457)/ % | pH(50 g/L 水溶液, 25 ℃) | w (Na2SO3)/ % | w (Na2CO3)/ % | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 无水Na2SO4 | 86.3 | 0.08 | — | 0.03 | 0.035 | 0.57 | 65 | 8.6 | 11.5 | 0.6 | ||
| 指 标 | Ⅱ类 | 一等品 | ≥98.0 | ≤0.10 | ≤0.30 | ≤0.70 | ≤0.010 | ≤0.50 | 82 | — | ||
| 合格品 | ≥97.0 | ≤0.20 | ≤0.40 | ≤0.90 | ≤0.040 | ≤1.0 | — | — | ||||
| Ⅲ类 | 一等品 | ≥95.0 | — | ≤0.60 | ≤2.0 | — | ≤1.5 | — | — | |||
| 合格品 | ≥92.0 | — | — | — | — | — | — | |||||
Table 3
Particle size distribution of products with different iron contents"
| 杂质Fe含 量/(mg·L-1) | 质量分数/% | 平均粒径/ μm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <75 μm | 75~125 μm | 125~150 μm | 150~212 μm | 212~300 μm | 300~425 μm | 425~600 μm | 600~850 μm | ||
| 0 | 2.32 | 5.98 | 6.39 | 13.11 | 25.11 | 27.98 | 19.01 | 0.10 | 303.23 |
| 11.1,无沉淀 | 3.78 | 8.54 | 7.01 | 12.76 | 21.33 | 29.56 | 17.02 | 0.00 | 291.68 |
| 20.0,有沉淀 | 7.21 | 11.57 | 12.05 | 13.56 | 18.87 | 34.43 | 2.31 | 0.00 | 240.34 |
| 50.0,有沉淀 | 8.11 | 11.72 | 13.25 | 12.27 | 17.89 | 35.44 | 1.32 | 0.00 | 236.22 |
Table 5
Particle size distribution of products with different impurity(Na2SO3) contents"
| 组成 | 质量分数/% | 平均粒径/ μm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <75 μm | 75~125 μm | 125~150 μm | 150~212 μm | 212~300 μm | 300~425 μm | 425~600 μm | 600~850 μm | ||
| Na2SO4 20.0% | 2.32 | 5.98 | 6.39 | 13.11 | 25.11 | 27.98 | 19.01 | 0.10 | 303.23 |
| Na2SO4 15.0%,Na2SO3 5.0% | 11.55 | 25.34 | 11.23 | 8.22 | 12.86 | 28.26 | 2.54 | 0.00 | 208.37 |
| Na2SO4 20.0%,Na2SO3 5.0% | 10.88 | 17.95 | 20.66 | 9.12 | 11.65 | 17.96 | 11.78 | 0.00 | 222.25 |
Table 7
Particle size distribution of products with different sample treatment methods"
| 样品处理方式 | 质量分数/% | 平均粒径/ μm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <75 μm | 75~125 μm | 125~150 μm | 150~212 μm | 212~300 μm | 300~425 μm | 425~600 μm | 600~850 μm | ||
| 样品20.0%,无处理 | 23.65 | 24.56 | 26.32 | 11.89 | 8.91 | 4.65 | 0.02 | 0.00 | 130.91 |
| 过滤除铁沉淀 | 18.56 | 21.83 | 30.11 | 12.65 | 10.40 | 5.43 | 1.02 | 0.00 | 144.62 |
| 氧化Na2SO3 | 8.79 | 13.99 | 14.12 | 13.23 | 13.22 | 24.11 | 12.54 | 0.00 | 246.16 |
Table 9
Anhydrous Na2SO4 product components before and after renovation project"
| 项目 | w (Na2SO4)/% | w(水不 溶物)/% | w[钙和镁 (以Mg计)]/% | w[氯化物 (以Cl计)]/% | w [铁(Fe)]/% | w (水分)/% | 白度 (R457)/% | pH(50 g/L水 溶液,25℃) | w (Na2SO3)/% | w (Na2CO3) /% |
|---|---|---|---|---|---|---|---|---|---|---|
| 改造前 | 86.3 | 0.08 | — | 0.03 | 0.035 | 0.57 | 65 | 8.6 | 11.5 | 0.6 |
| 改造后 | 98.4 | 0.09 | — | 0.03 | 0.005 | 0.42 | 82 | 7.5 | 0.5 | 0.4 |
Table 10
Particle size distribution of anhydrous Na2SO4 products and industrial anhydrous Na2SO4 before and after renovation project"
| 组成 | 质量分数/% | 平均粒径/ μm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <75 μm | 75~125 μm | 125~150 μm | 150~212 μm | 212~300 μm | 300~425 μm | 425~600 μm | 600~850 μm | ||
| 改造前无水Na2SO4 | 28.11 | 49.76 | 8.21 | 5.87 | 5.01 | 2.61 | 0.43 | 0.00 | 106.71 |
| 改造后无水Na2SO4 | 13.98 | 44.33 | 18.76 | 11.49 | 7.35 | 3.97 | 0.12 | 0.00 | 129.99 |
| 工业无水Na2SO4 | 7.78 | 41.20 | 26.54 | 21.58 | 2.65 | 0.25 | 0.00 | 0.00 | 127.36 |
| [1] | 陆操, 程荫东. 无水硫酸钠生产、消费及对策[J]. 无机盐工业, 1992(3):14-17. |
| [2] | 李淑萍. 大颗粒无水硫酸钠结晶工艺及数学模型研究[D]. 太原:华北工学院, 2001. |
| [3] | 廖恩鑫, 陈丽芳, 张泽亚, 等. 硫酸钠与氯化钾制备硫酸钾实验研究[J]. 无机盐工业, 2020, 52(10):106-109. |
| [4] | 谢明胜, 卫锋, 王爱广. 颗粒状无水硫酸钠的生产[J]. 无机盐工业, 2003, 35(6):30-31. |
| [5] | 刘云琴. 元明粉结块原因及防止方法的探讨[J]. 无机盐工业, 2001, 33(2):28-29. |
| [6] | 唐娜, 白丽荣, 沙作良, 等. 添加剂对硫酸钠晶体粒度的影响[J]. 盐业与化工, 2007, 36(6):1-3. |
| [7] | 黄欣, 陈业钢, 苏楠楠, 等. 高盐废水分质结晶及资源化利用研究进展[J]. 化学工业与工程, 2019, 36(1):10-23. |
| [8] | 裴旭东, 陈卫红, 李朝恒. 煤化工废水中硫酸钠-氯化钠-硝酸钠分离工艺研究[J]. 工业水处理, 2020, 40(1):63-66. |
| [9] | 雷少成, 李玉林. DTNF膜对煤化工HERO高盐废水的分盐实验研究[J]. 无机盐工业, 2020, 52(9):84-87. |
| [10] |
BHARMORIA P, GEHLOT P S, GUPTA H, et al. Temperaturedependent solubility transition of Na2SO4 in water and the effect of NaCl therein:solution structures and salt water dynamics[J]. Journal of Physical Chemistry B, 2014, 118(44):12734-12742.
doi: 10.1021/jp507949h |
| [11] | 张峰榛, 张孝果, 杨虎, 等. 硫酸钠废水真空蒸发结晶脱盐性能研究[J]. 天然气化工:C1化学与化工, 2019, 44(3):49-52. |
| [12] | 许莉, 丁成荣. 铁离子杂质对过硫酸钠结晶过程的影响[J]. 精细化工, 2001, 18(9):547-549. |
| [13] |
ZENG G, LI H, LUO S, et al. Effects of ultrasonic radiation on induction period and nucleation kinetics of sodium sulfate[J]. Korean Journal of Chemical Engineering, 2014, 31(5):807-811.
doi: 10.1007/s11814-013-0290-6 |
| [14] |
SU N, WANG Y, YAN X, et al. Mechanism of influence of organic impurity on crystallization of sodium sulfate[J]. Industrial & Engineering Chemistry Research, 2018, 57(5):1705-1713.
doi: 10.1021/acs.iecr.7b04625 |
| [15] | 曾桂生, 李慧, 王贤勇. 无水硫酸钠结晶热力学及介稳区宽度研究[J]. 南昌航空大学学报:自然科学版, 2012, 26(4):66-70. |
| [16] | 丁爱娟. 亚熔盐法粉煤灰提铝清洁工艺硫行为研究[D]. 北京:北京化工大学, 2013. |
| [17] | 侯长军, 霍丹群, 唐晓萍. 结晶添加剂对硫酸钾结晶过程影响研究[J]. 海湖盐与化工, 2002, 31(2):20-24. |
| [18] | 丁绪淮, 谈遒. 工业结晶[M]. 北京: 化学工业出版社, 1985:74-132. |
| [19] | 科尔索夫. 定量化学分析[M]. 南京化工学院分析化学教研组译. 北京: 人民教育出版社, 1981:234-235. |
| [1] | WANG Lijuan, YAN Kezhou, GUO Zhiqiang, ZHAO Zhonghe, GUO Yanxia, CHENG Fangqin. Preparation of poly-aluminum chloride from acid leaching liquor of red mud-coal gangue activated by sodium salt [J]. Inorganic Chemicals Industry, 2023, 55(4): 76-83. |
| [2] | WU Hao,LI Xi,ZHANG Jun,DUAN Siyu. Study on impurity removal process of prereduction-oxalic acid precipitation in stainless steel coloring ageing liquid [J]. Inorganic Chemicals Industry, 2023, 55(2): 119-125. |
| [3] | ZHU Wanye,TANG Ding,CHI Heting,LIAO Xianghui,ZHUANG Rongchuan,WANG Qiankun,SHEN Qingfeng. Study on impurity removal rule of ferrous sulfate from by-product of titanium dioxide by crystallization purification [J]. Inorganic Chemicals Industry, 2022, 54(7): 105-109. |
| [4] | GU Qingshan,LIN Xihua,Zhao Shihao,YUAN Yijin. Effect of different pretreatment processes on properties of phosphogypsum [J]. Inorganic Chemicals Industry, 2022, 54(4): 17-23. |
| [5] | Wu Zhaoyang,Zhang Yongxing,Zhang Lizhen,Zhang Xiufeng. Effect of phase composition and impurity content of β-gypsum on its properties [J]. Inorganic Chemicals Industry, 2021, 53(9): 67-71. |
| [6] | Tan Bo,Liu Xianghuan,Liu Xudong,Yi Meigui. Study on law of lithium extraction and impurity removal from spodumene leaching solution [J]. Inorganic Chemicals Industry, 2021, 53(4): 56-60. |
| [7] | An Xiaoying,Huang Wengfang,Wang Zhengli,Peng Yihua,Ye Jiasheng,Li Ming,Lai Xiaoli. Discussion on determination method of zinc constant in waste chemicals [J]. Inorganic Chemicals Industry, 2021, 53(3): 84-86. |
| [8] | Cao Liqiong,Zhang Xiaoxi,Wu Lixiang,Cheng Huaigang,Cheng Fangqin. Experimental investigation of floating phenomenon of carnalliti NaCl in direct flotation process of KCl [J]. Inorganic Chemicals Industry, 2020, 52(7): 26-29. |
| [9] | Han Longnian,Xin Jing,Zhang Ping,Chen Yufei,Fan Wenxuan,Wei Linlin. Analysis of impurity deposition on guard catalyst for diesel hydrocracking device [J]. Inorganic Chemicals Industry, 2020, 52(12): 113-117. |
| [10] | Huang Zhong,Yu Shuangqiang,Gao Kaiyuan,He Jun,Huang He,Zhang Xianfeng,Chen Xizhen. Study on process technology of preparing NH4F and co-producing MgF2 with high impurity fluosilicic acid [J]. Inorganic Chemicals Industry, 2020, 52(10): 110-116. |
| [11] | Fan Tianbo,Jiang Yu,Chen Si,You Gang,Ding Ke,Sun Xiaojun,Liu Luping,Li Li,Liu Yunyi,Hu Kaizhou,Ma Junru,Guo Xiyao. Application of TRIZ theory in preparation of magnesium hydroxide by light burning ammonia [J]. Inorganic Chemicals Industry, 2019, 51(5): 23-27. |
| [12] | LIU Qiu-Bin, CHEN Yi, WU Bo-Rong, CHEN Fei-Biao. Study on preparing high purity manganese chloride from electrolytic manganese powder [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(6): 20-. |
| [13] | LI Xue, ZHANG Ying, HOU Rui, ZHANG Yan, LIU Yun-Yi. Influence of impurity ions on magnesium hydroxide product in ammonia-evaporation refined solution [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(10): 23-. |
| [14] | WANG Yuan-Wang, YANG Ming-Ping, YAO Mao-Sheng, WANG Yuan-Hong. Study on preparation technology of high purity potassium metavanadate [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(10): 40-. |
| [15] | WANG Bin, LI Chun, YANG Yang-Jun. Distribution of impurities from low grade titanium slag as raw material in titanium dioxide manufacturing by sulfate process [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(1): 35-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||