Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (1): 77-82.doi: 10.19964/j.issn.1006-4990.2021-0163
• Environment·Health·Safety • Previous Articles Next Articles
Study on kinetics of phosphate tailings calcination and acid leaching process
ZHOU Jiaqi( ),CHEN Kui(
),CHEN Kui( ),WU Bin,JI Lijun,WU Yanyang
),WU Bin,JI Lijun,WU Yanyang
- School of Chemical Engineering,East China University of Science and Technology,Shanghai 200237,China
-
Received:2021-03-18Online:2022-01-10Published:2022-03-14 -
Contact:CHEN Kui E-mail:jiaqizhou77@163.com.cn;chenkui@ecust.edu.cn
CLC Number:
Cite this article
ZHOU Jiaqi,CHEN Kui,WU Bin,JI Lijun,WU Yanyang. Study on kinetics of phosphate tailings calcination and acid leaching process[J]. Inorganic Chemicals Industry, 2022, 54(1): 77-82.
share this article
Table 3
α-T data of thermal decomposition of dolomite in phosphorus tailings at different heating rates"
| 反应 分解率 | 热分解温度点/K | |||
|---|---|---|---|---|
| 5 K/min | 10 K/min | 15 K/min | 20 K/min | |
| 0.1 | 876 | 912 | 920 | 919 |
| 0.2 | 928 | 974 | 979 | 980 |
| 0.3 | 951 | 997 | 1 000 | 1 004 |
| 0.4 | 967 | 1 010 | 1 011 | 1 018 |
| 0.5 | 978 | 1 020 | 1 021 | 1 029 |
| 0.6 | 987 | 1 031 | 1 033 | 1 039 |
| 0.7 | 994 | 1 041 | 1 042 | 1 048 |
| 0.8 | 1 001 | 1 049 | 1 051 | 1 058 |
| 0.9 | 1 008 | 1 057 | 1 059 | 1 068 |
| 1.0 | 1 272 | 1 271 | 1 232 | 1 264 |
Table 4
Fitting results of kinetic parameters of dolomite thermal decomposition in phosphorus tailings"
| β/ (K·min-1) | 模型 序号 | E/ (kJ·mol-1) | ln A | R2 | 模型 序号 | E/ (kJ·mol-1) | ln A | R2 | 模型 序号 | E/ (kJ·mol-1) | ln A | R2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 1 | 205.996 | 23.068 7 | 0.955 0 | 4 | 57.935 | 3.738 3 | 0.938 9 | 7 | 372.306 | 41.369 8 | 0.976 3 |
| 2 | 310.632 | 36.918 0 | 0.883 1 | 5 | 39.427 | 1.095 7 | 0.927 2 | 8 | 165.480 | 16.934 2 | 0.996 5 | |
| 3 | 94.950 | 8.747 8 | 0.947 9 | 6 | 281.236 | 31.513 2 | 0.982 1 | 9 | 178.105 | 18.231 5 | 0.974 3 | |
| 10 | 1 | 222.211 | 24.518 1 | 0.976 1 | 4 | 62.811 | 4.675 0 | 0.967 8 | 7 | 399.895 | 43.236 3 | 0.990 2 |
| 2 | 337.876 | 39.096 2 | 0.916 4 | 5 | 42.887 | 1.971 0 | 0.961 8 | 8 | 177.633 | 18.124 3 | 0.993 8 | |
| 3 | 102.661 | 9.811 2 | 0.972 4 | 6 | 300.066 | 32.845 2 | 0.997 2 | 9 | 191.503 | 19.500 0 | 0.989 4 | |
| 15 | 1 | 232.409 | 26.101 2 | 0.985 5 | 4 | 66.169 | 5.505 3 | 0.980 7 | 7 | 417.146 | 45.597 0 | 0.995 2 |
| 2 | 354.270 | 41.377 8 | 0.934 4 | 5 | 45.389 | 2.710 9 | 0.977 3 | 8 | 185.512 | 19.445 7 | 0.997 1 | |
| 3 | 107.729 | 10.827 6 | 0.983 3 | 6 | 312.251 | 34.635 6 | 0.993 9 | 9 | 200.098 | 20.902 2 | 0.994 8 | |
| 20 | 1 | 215.588 | 24.188 5 | 0.980 7 | 4 | 60.515 | 4.989 6 | 0.973 8 | 7 | 388.331 | 42.168 3 | 0.992 2 |
| 2 | 328.412 | 38.330 5 | 0.926 9 | 5 | 41.131 | 2.362 4 | 0.968 7 | 8 | 172.139 | 17.966 4 | 0.994 8 | |
| 3 | 99.283 | 9.966 8 | 0.977 6 | 6 | 291.095 | 32.170 4 | 0.994 8 | 9 | 185.655 | 19.288 4 | 0.991 6 |
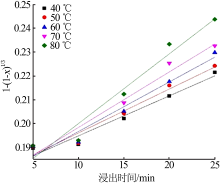
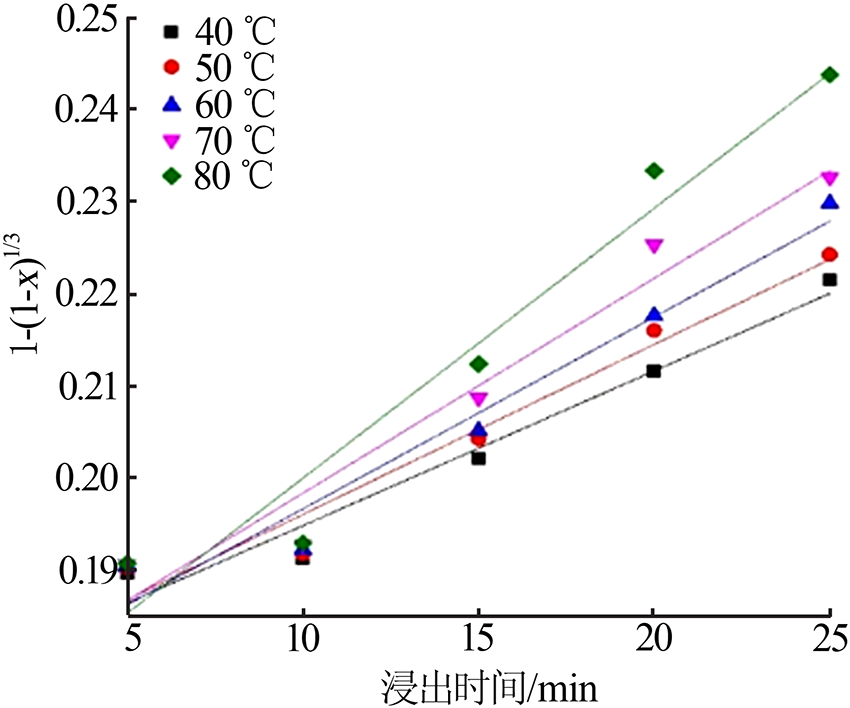
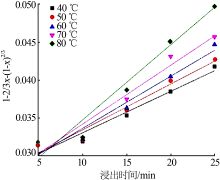
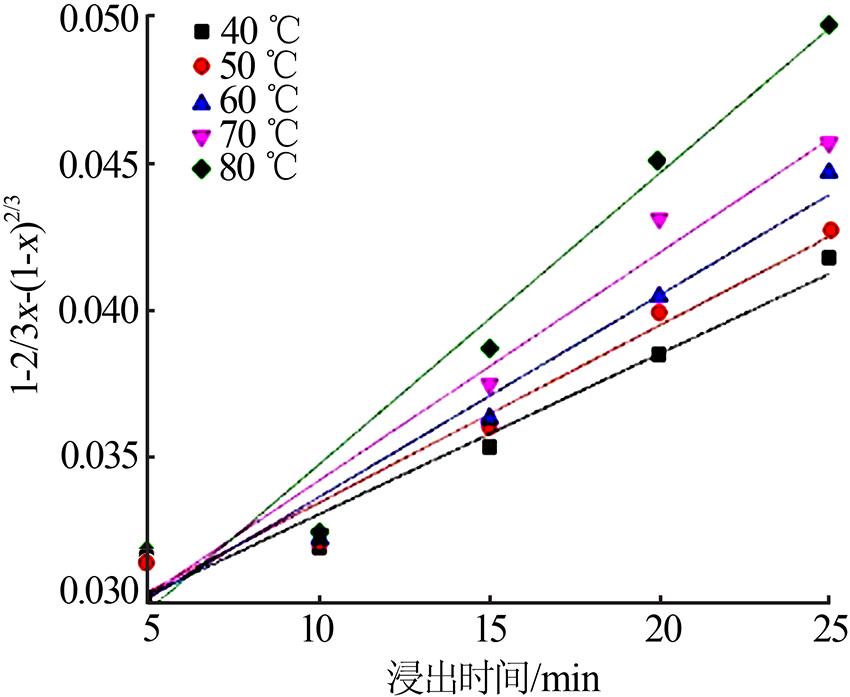
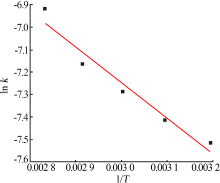
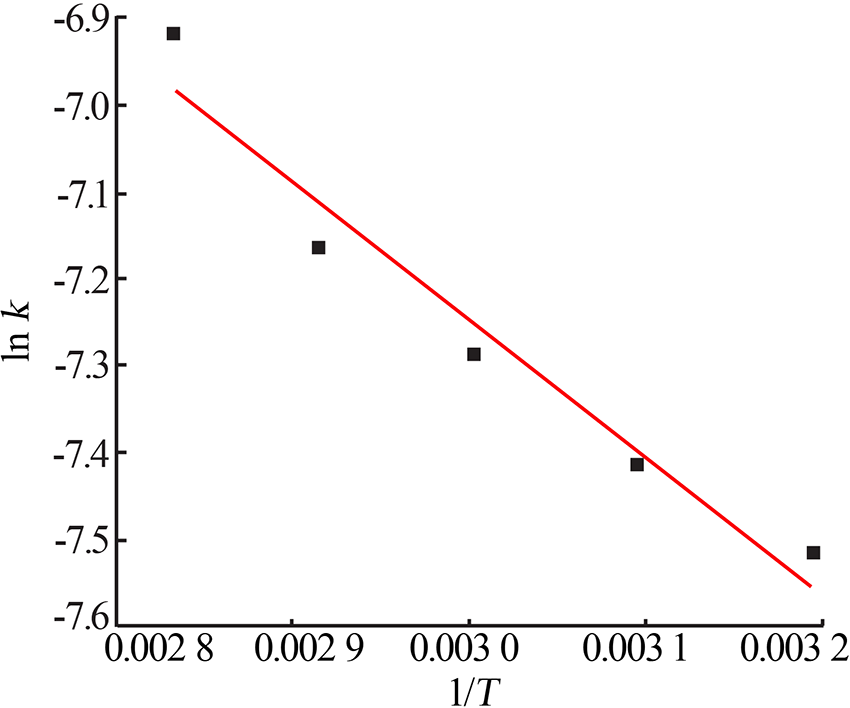
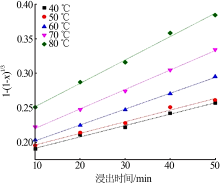
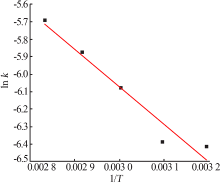
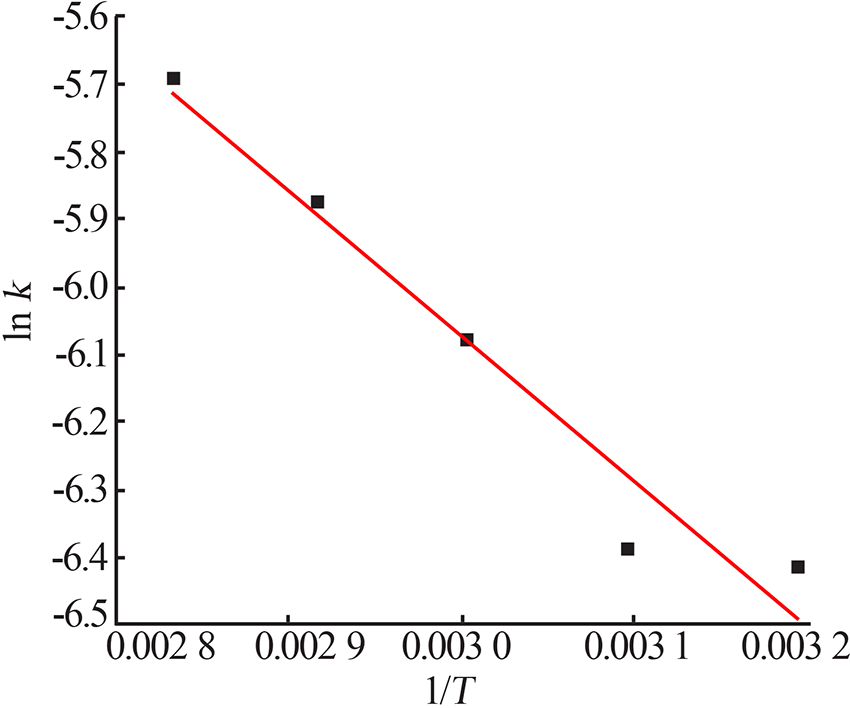
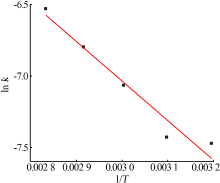
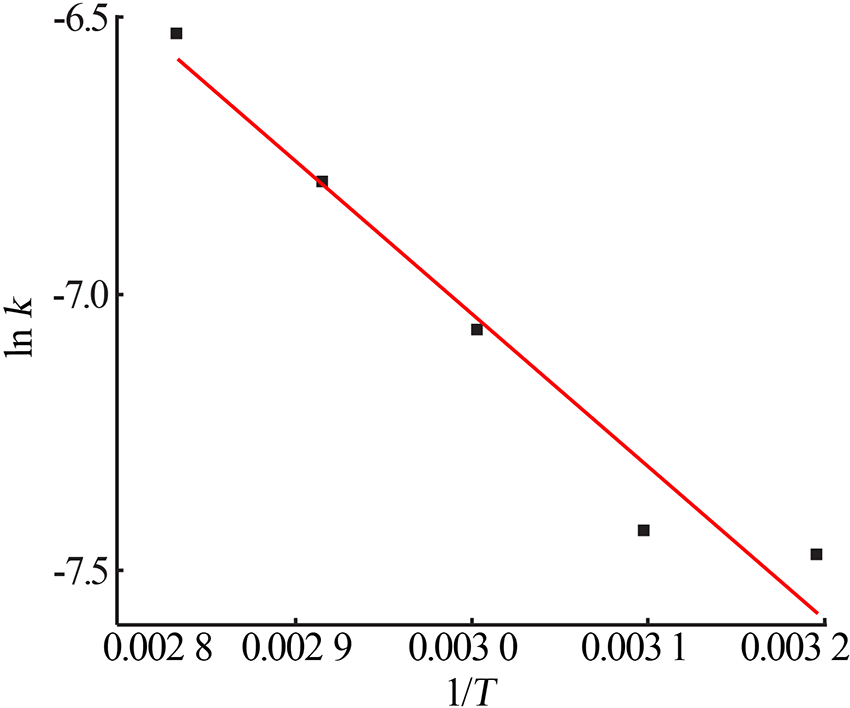
Table 5
Fitting result of chemical reaction model and diffusion control model"
| 反应 温度/K | 化学反应控制模型 | 内扩散控制模型 | ||
|---|---|---|---|---|
| k1/10-1 | R12 | k2/10-3 | R22 | |
| 313 | 0.016 8 | 0.950 9 | 0.544 9 | 0.947 5 |
| 323 | 0.018 4 | 0.949 4 | 0.602 7 | 0.947 7 |
| 333 | 0.020 8 | 0.947 3 | 0.685 7 | 0.942 6 |
| 343 | 0.023 3 | 0.941 7 | 0.773 8 | 0.940 6 |
| 353 | 0.029 2 | 0.940 4 | 0.988 8 | 0.938 3 |
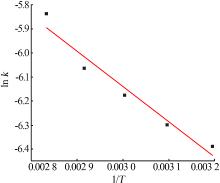
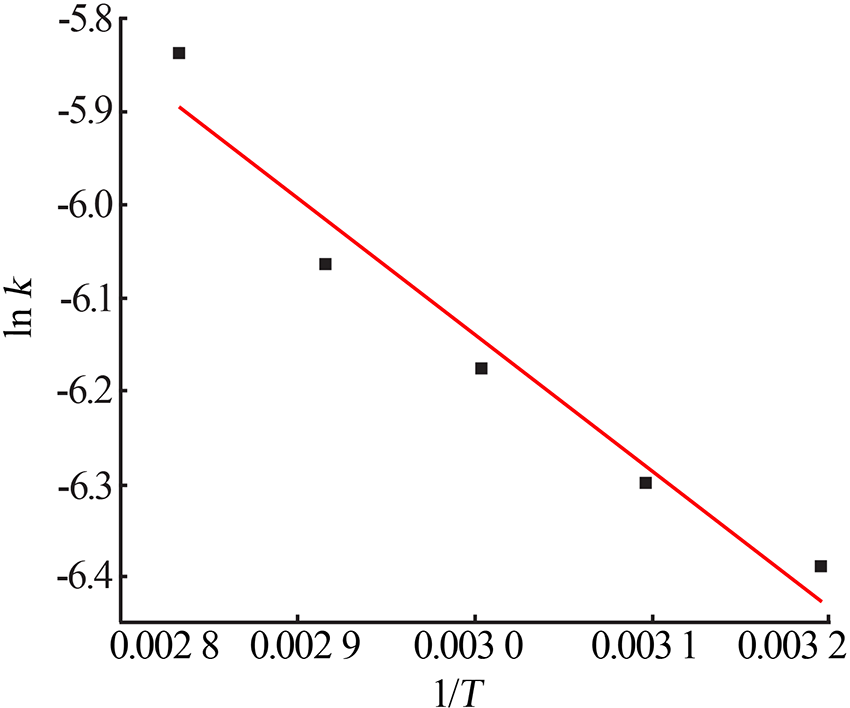
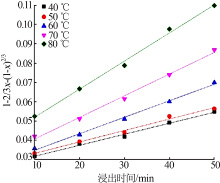
Table 7
Fitting result of chemical reaction model and diffusion control model"
| 反应 温度/K | 化学反应控制模型 | 内扩散控制模型 | ||
|---|---|---|---|---|
| k1/10-1 | R12 | k2/10-3 | R22 | |
| 313 | 0.016 4 | 0.990 9 | 0.569 8 | 0.990 7 |
| 323 | 0.016 8 | 0.986 5 | 0.592 4 | 0.986 8 |
| 333 | 0.022 9 | 0.998 9 | 0.853 5 | 0.994 5 |
| 343 | 0.028 1 | 0.997 9 | 1.120 | 0.992 6 |
| 353 | 0.033 7 | 0.994 5 | 1.460 | 0.993 9 |
| [1] | 俞政一. 磷矿物和磷矿石的性质及分类[J]. 硫磷设计, 1998(2):29-37. |
| [2] | 吴汉军. 磷尾矿综合利用研究[D]. 武汉:武汉工程大学, 2015. |
| [3] | 马一嘉, 武俊杰, 倪天阳. 我国磷矿资源的开发利用现状及进展[J]. 矿冶, 2018, 27(2):53-56. |
| [4] | 甄逢生, 沙惠雨, 刘长淼, 等. 中国磷矿石选矿工艺研究现状[J]. 金属矿山, 2018(2):7-13. |
| [5] | 胡宏, 李艳. 高镁磷尾矿渣制取磷镁复合肥料的试验研究[J]. 贵州化工, 2009, 34(2):20-21,25. |
| [6] | 刘啸龙. 白肥中磷的回收利用及酸解动力学研究[D]. 上海:华东理工大学, 2015. |
| [7] | 吴礼定, 曾波, 王生军, 等. 中低品位磷矿尾矿的综合利用研究进展[J]. 云南化工, 2008(35):55-58. |
| [8] |
RUAN Renman, WEN Jiankang, CHEN Jinghe. Bacterial heap-each-ing:Practice in zijinshan copper mine[J]. Hydrometallurgy, 2006, 83(1/4):77-82.
doi: 10.1016/j.hydromet.2006.03.048 |
| [9] | 赵鑫, 蔡慢弟, 董倩倩, 等. 中低品位磷矿资源高效利用机制与途径研究进展[J]. 植物营养与肥料学报, 2018, 24(4):1121-1130. |
| [10] | 刘润哲, 刘丽芬, 欧志兵, 等. 磷矿尾矿资源化利用研究进展[J]. 化工矿物与加工, 2020, 49(2):52-56. |
| [11] | 周坤, 唐云, 张群, 等. 磷尾矿制备磷酸铵镁试验研究[J]. 矿业研究与开发, 2015, 35(4):31-33. |
| [12] | 孙娜, 尤彩霞. 磷尾矿制备氢氧化镁研究进展[J]. 河南科技, 2019(10):126-127. |
| [13] | 丁双双, 李燕婷, 袁亮, 等. 小分子有机物螯合钙肥对樱桃番茄产量、品质和养分吸收的影响[J]. 中国土壤与肥料, 2015(5):61-66. |
| [14] | 张景华, 郭亚楠, 徐维辰, 等. 山梨糖醇钙离子螯合物稳定性的探究[J]. 山东化工, 2017, 46(13):14-16. |
| [15] | ZHANG Y, FU C, YAN Y, et al. Zinc sulfate and sugar alcohol zinc sprays at critical stages to improve apple fruit quality[J]. Horttech-nology, 2013, 23(4):490-497. |
| [16] | 周飞, 杨林, 曹建新. 中低品位磷矿焙烧过程中白云石热分解动力学研究[J]. 硅酸盐通报, 2019, 38(5):1377-1383,1389. |
| [17] | 章佳豪, 郁青春, 杨斌, 等. 白云石热分解动力学机理探究[J]. 有色金属工程, 2020, 10(12):55-61. |
| [18] | 郑峰伟. 粉煤灰中铝铁浸出过程及动力学研究[D]. 昆明:昆明理工大学, 2018. |
| [19] | TENG Qing, FENG Yali, MA Ying. Study on acid leaching kinetics of magnesite tailings[J]. Metal Mine, 2017(11):189-193. |
| [20] | 王海波, 吴小平, 高健, 等. 硫酸浸取钛铁矿动力学研究[J]. 钢铁钒钛, 2020, 41(6):6-10. |
| [21] | 张汉泉, 蔡祥, 陈官华, 等. 含铜硫酸渣中铜的浸出及浸出动力学分析[J]. 有色金属工程, 2020, 10(4):44-50,56. |
| [1] | WANG Shengchang, HAO Jianying, CHEN Jianing, TIAN Bo. Effect of calcination on properties of calcined gypsum prepared from desulphurization gypsum with potassium aluminum sulfate [J]. Inorganic Chemicals Industry, 2024, 56(4): 105-111. |
| [2] | QIAN Zhihui, ZHU Qin, MA Jiao, GUO Yujiao, XIANG Mingwu, GUO Junming. Study on preparation and electrochemical properties of nano-sized LiNi0.05Mn1.95O4 cathode materials [J]. Inorganic Chemicals Industry, 2024, 56(4): 50-56. |
| [3] | YU Zhou, HE Zhaoyi, TANG Liang, HE Sheng, XIAO Haixin, XIAO Yixun. Study on preparation and microscopic properties of typical sulfate solid waste composite cementitious materials [J]. Inorganic Chemicals Industry, 2024, 56(4): 90-97. |
| [4] | WANG Jianrui, ZHANG Song, ZHANG Jie. Study on new process for beneficiation phosphate of low-grade phosphate rock leaching via lactic acid [J]. Inorganic Chemicals Industry, 2024, 56(3): 56-63. |
| [5] | WANG Ruting, ZHAO Xiaorong, HUANG Xuquan, WANG Haojie, XUE Fei, CAI Jiawei. Research on preparation and early performance of mixed phase phosphogypsum-based cementing materials [J]. Inorganic Chemicals Industry, 2024, 56(3): 98-104. |
| [6] | HUANG Tao, HUANG Zili, XIAO Shuo, ZHENG Jiemiao, LIU Xiaofeng, WU Jilong. Experimental study on preparation of polyferric chloride from iron tailings acid leaching solution [J]. Inorganic Chemicals Industry, 2024, 56(2): 121-126. |
| [7] | YANG Kun, REN Qixia, DONG Yonggang, LIU Fei, YAO Mengqin, CAO Jianxin. Effect of calcination temperature on catalytic performance of ZnGaZrO x /SAPO-34 [J]. Inorganic Chemicals Industry, 2024, 56(2): 136-145. |
| [8] | XIA Guiying, YANG Liuchun, YUAN Zhiye. Study on direct leaching of rare earth elements from phosphogypsum with sulfuric acid [J]. Inorganic Chemicals Industry, 2024, 56(1): 107-113. |
| [9] | XIANG Mengqi, MENG Hua, WANG Ye, MENG Xianzhang, BAI Yuhang, WANG Yujunyao, ZHANG Yidan. Study on kinetic of iron leaching from titanium gypsum and its cyclic acid leaching process [J]. Inorganic Chemicals Industry, 2024, 56(1): 114-120. |
| [10] | ZHANG Conghua, YAN Wenbin, XIAO Jiajun, ZHAO Ke, PENG Shangquan, WEI Yuhong. Reductive leaching technology of manganese anode slag using tartaric acid as reducing agent optimized by RSM [J]. Inorganic Chemicals Industry, 2023, 55(9): 106-113. |
| [11] | LI Qiang, YOU Xiaomin, SHE Xuefeng, JIANG Zeyi, XUE Qingguo, WANG Jingsong. Effect of calcination temperature and carbon structure on compressive strength of CaO-containing carbon pellets [J]. Inorganic Chemicals Industry, 2023, 55(9): 43-49. |
| [12] | LUO Wenbo, LI Heng, LÜ Jun, YANG Linguang, ZHAO Xingfan, LONG Xiao. Study on recovery of silicon and aluminum from industrial silicon slag [J]. Inorganic Chemicals Industry, 2023, 55(9): 94-99. |
| [13] | FAN Fangfang, TONG Zhongkai, ZUO Weiyuan. Study on adsorption of tetracycline from wastewater by calcium modified peanut shell biochar [J]. Inorganic Chemicals Industry, 2023, 55(6): 109-115. |
| [14] | LI Heng, ZHANG Hui, ZI Xuemin. Analysis on calcination process progress of phosphogypsum [J]. Inorganic Chemicals Industry, 2023, 55(6): 27-35. |
| [15] | DONG Xinyu, WANG Haifeng, HE Yue, YANG Pan, WANG Song, YANG Chunyuan, WANG Qin, HUANG Bifang, WANG Jiawei. Exleaching toxicity analysis and harmless treatment of electrolytic manganese residue [J]. Inorganic Chemicals Industry, 2023, 55(5): 85-90. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||