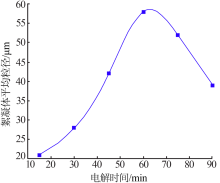
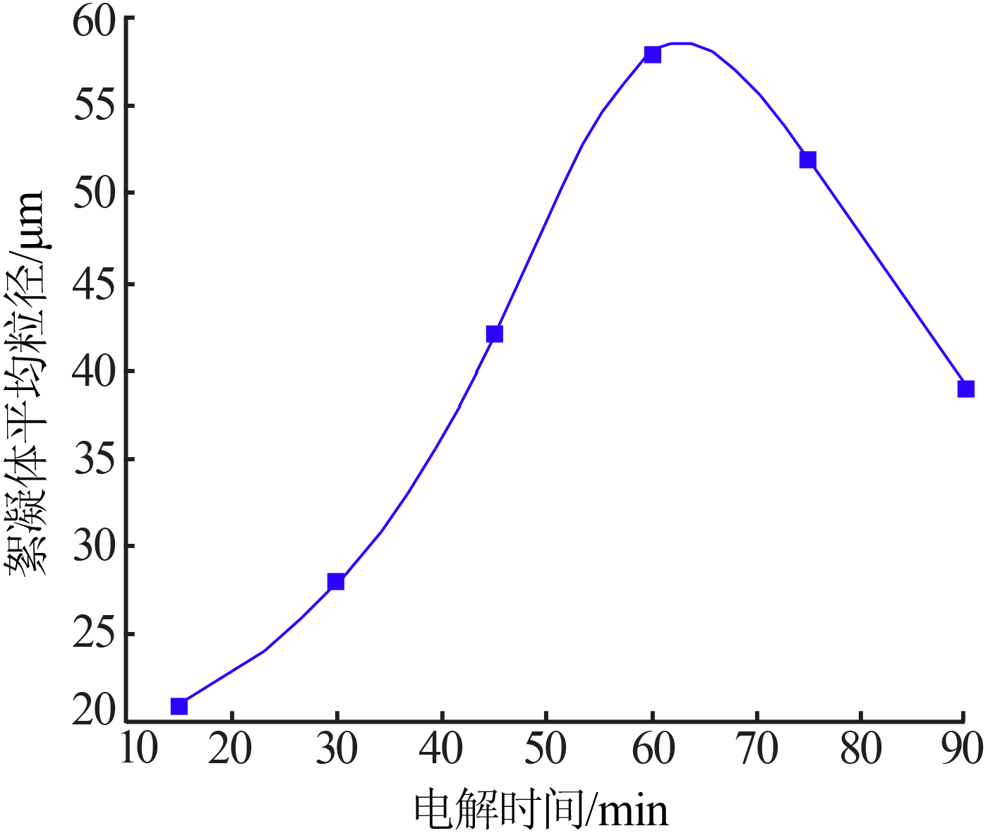
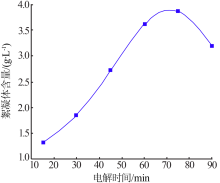
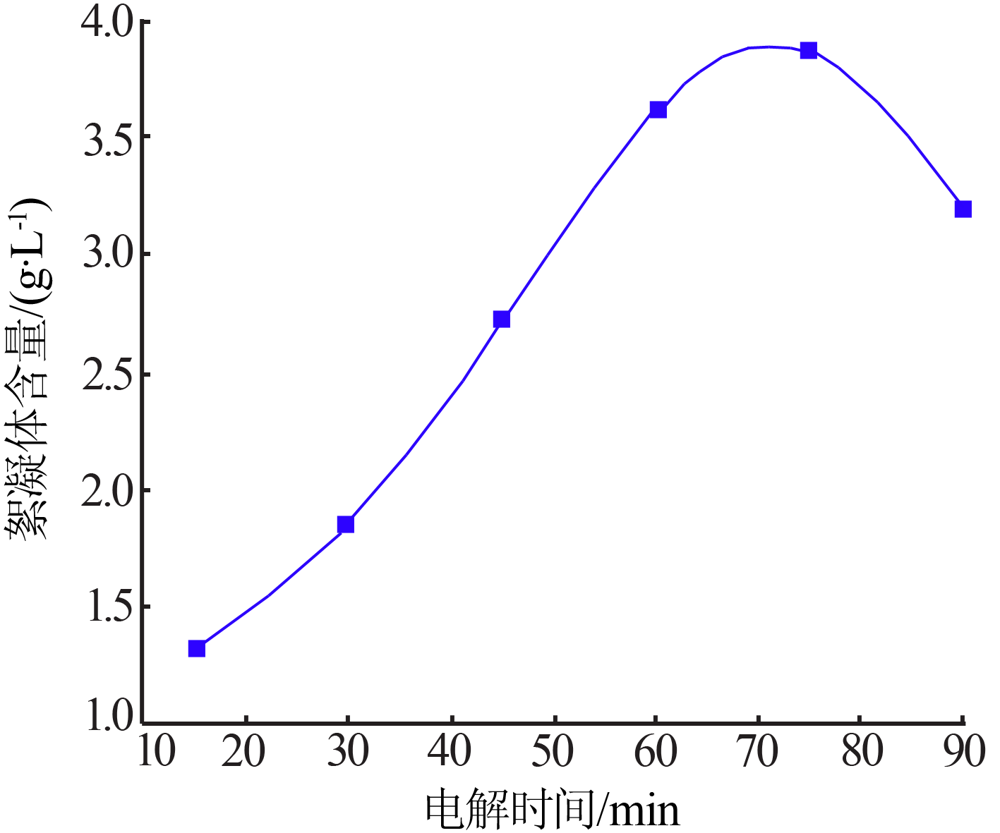
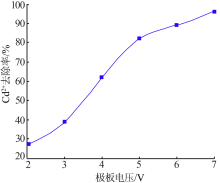
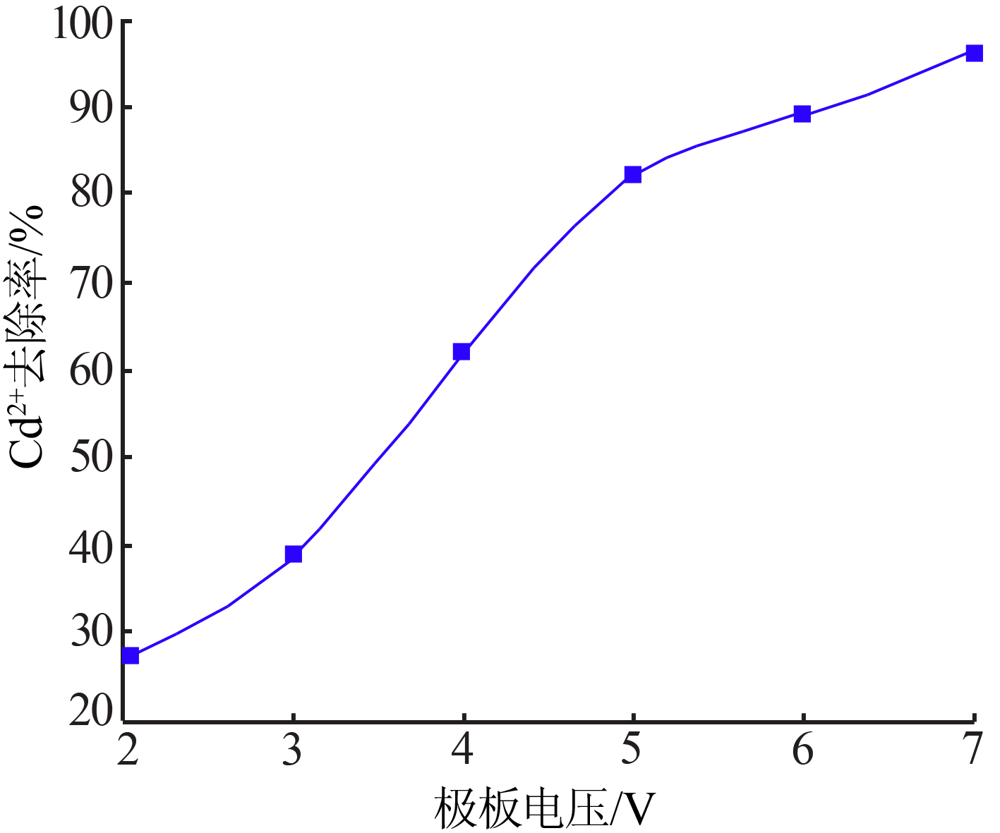
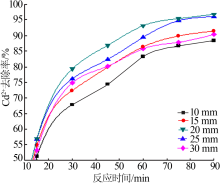
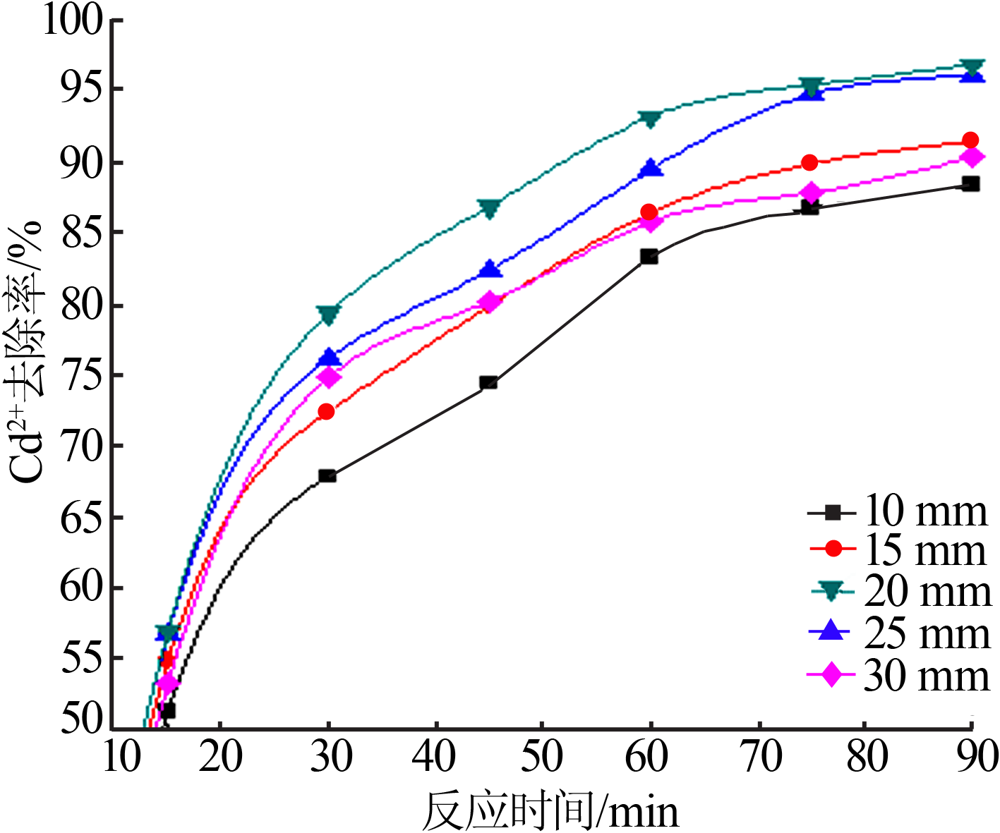
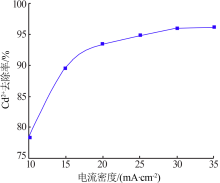
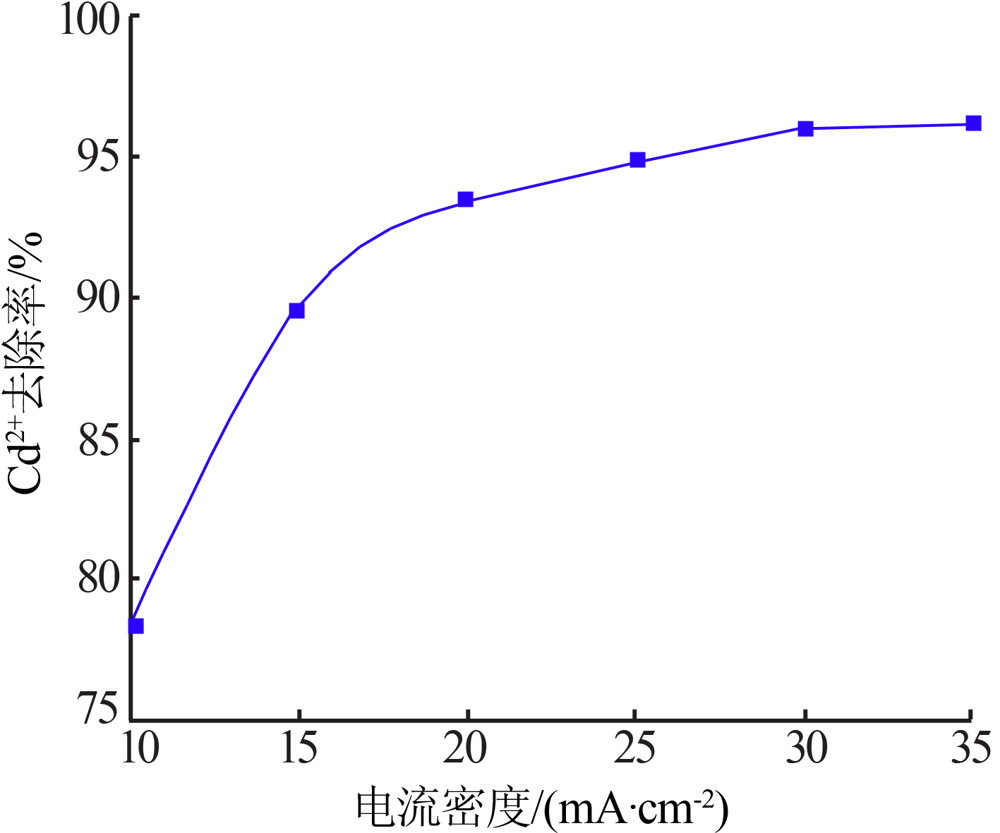
| [1] |
刘强, 林乃明, 沙春鹏, 等. 钢铁材料电镀镉的研究现状[J]. 表面技术, 2017, 46(1):146-157.
|
| [2] |
张厚. 电镀镉废水处理工艺研究[D]. 贵州: 贵州大学, 2019.
|
| [3] |
邹路易, 徐西腾, 李磊, 等. 聚二硫代氨基甲酸盐捕集剂处理含镉废水的研究[J]. 应用化工, 2015, 44(8):1383-1386.
|
| [4] |
韦刘勋. 锌铟冶炼企业镉废水污染及处理[J]. 科技风, 2018(4):102.
|
| [5] |
张汉鑫, 李慧, 梁精龙, 等. 有色冶炼废渣中有价金属回收的冶金方法应用之综述[J]. 中国钨业, 2018, 33(4):69-73.
|
| [6] |
张条兰, 刁润丽, 方秀苇. 电絮凝法处理电镀废水的研究进展[J]. 电镀与精饰, 2016, 38(3):33-37.
|
| [7] |
刘艳. 铝钛电极电絮凝法处理造纸废水工艺及机理的研究[D]. 陕西: 陕西科技大学, 2015.
|
| [8] |
温晓东, 杨盛春, 邓庆文, 等. 分离富集技术在紫外-可见分光光度法测定金属元素中的应用[J]. 大理大学学报, 2019, 12(4):36-43.
|
| [9] |
李影影. 铁板电絮凝法去除废水中PFOA、PFOS钾盐的研究[D]. 深圳: 深圳大学, 2015.
|
| [10] |
党亚攀. Fe-Al电极组合电絮凝法处理含Ni-EDTA废水的研究[D]. 广州: 华南理工大学, 2017.
|
| [11] |
孔令海, 刘家富, 杨春, 等. 芬顿氧化法处理氨羧配位剂电镀镉废水[J]. 电镀与涂饰, 2017, 36(12):651-654.
|
| [12] |
焦跃腾, 顾正华, 丁昊, 等. 板间距对电絮凝处理微污染水的影响[J]. 人民长江, 2017, 48(21):23-28.
|