Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (3): 48-53.doi: 10.11962/1006-4990.2020-0216
• Research & Development • Previous Articles Next Articles
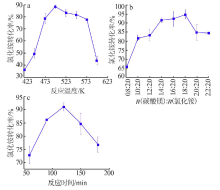
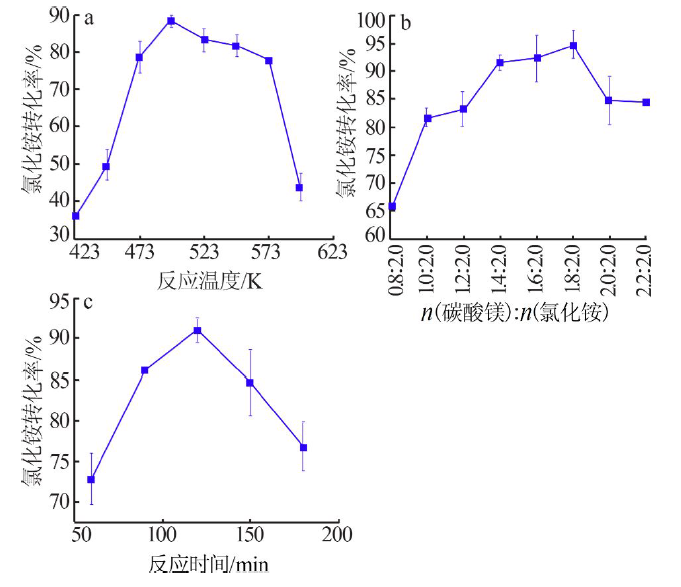
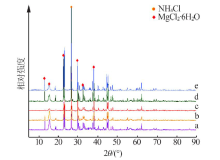
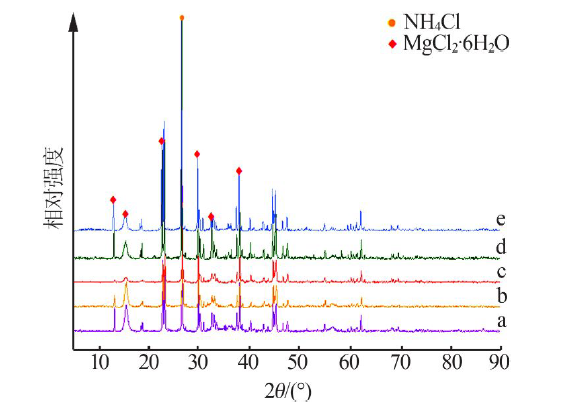
Study on mechanism and conditions of the new process of ammonium chloride conversion
Li Fei1,2,3( ),Zhou Jianmin1,Li Chengyu1,Xu Wentao1,Zhang Xinyue1,Li Xinwei1,Wang Jun1,2,3,Ji Zhiyong1,2,3,Zhao Yingying1,2,3,Guo Xiaofu1,2,3,Yuan Junsheng1,2,3(
),Zhou Jianmin1,Li Chengyu1,Xu Wentao1,Zhang Xinyue1,Li Xinwei1,Wang Jun1,2,3,Ji Zhiyong1,2,3,Zhao Yingying1,2,3,Guo Xiaofu1,2,3,Yuan Junsheng1,2,3( )
)
- 1. School of Chemical Engineering,Hebei University of Technology,Engineering Research Center for Chemical Technology of the Efficient Utilization of Seawater Resources,Tianjin 300130,China
2. Collaborative Innovation Center of Hebei Modern Marine Chemical Technology
3. School of Chemical Engineering,Hebei University of Technology,Chemical Energy Conservation Process Integration and Resource Utilization National-Local Joint Engineering Laboratory