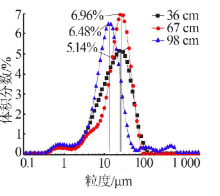
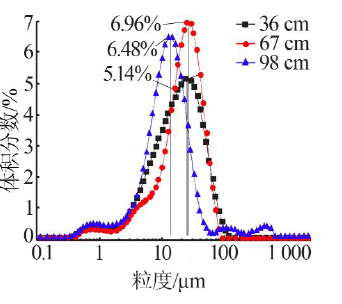
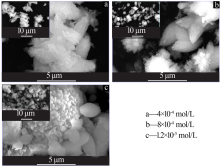
| [1] |
Jacek W, Agnieszka R. Donnan dialysis for hardness removal from water before electrodialytic desalination[J]. Desalination, 2007,212(1/2/3):251-260.
doi: 10.1016/j.desal.2006.11.008
|
| [2] |
孔祥波. 超微细无定形碳酸钙粉体的制备、改性及其应用[D]. 厦门:厦门大学, 2017.
|
| [3] |
杨仲尼, 李增杰, 李帅. 纳米碳酸钙对道路石油沥青流变特性的影响[J].中外公路,2019(4):254-258.
|
| [4] |
梁永兰. 碳酸钙与鲑鱼降钙素协同治疗内分泌失调型骨质疏松症的临床疗效观察[J]. 心理月刊, 2019,14(15):187.
|
| [5] |
黄宸, 郝同辉, 张群朝, 等. 碳酸钙对PVC涂料流变与力学性能的影响[J]. 胶体与聚合物, 2019,37(1):14-16.
|
| [6] |
童鹏程, 杨松伟, 钱庆荣, 等. 加工方式对纳米碳酸钙母料的制备及其聚乳酸复合材料性能的影响[J]. 中国塑料, 2019,33(3):72-77.
|
| [7] |
赵红涛, 王树民, 刘志江, 等. 磷石膏矿化固定CO2制备高纯高白CaCO3[J].材料导报, 2019(18):3031-3034.
|
| [8] |
罗东山, 彭同江, 孙红娟, 等. 大理石尾矿碳化法制备文石晶须及其形貌分析[J]. 矿产保护与利用, 2019,39(4):60-65.
|
| [9] |
蒋余花, 孔凡滔, 马新胜. 氯化镁-氢氧化钠复合添加剂制备文石型碳酸钙[J]. 无机盐工业, 2016,48(12):44-48.
|
| [10] |
鲁月. 聚天冬氨酸与镁离子复合调控碳酸钙沉淀过程研究[D]. 哈尔滨:黑龙江大学, 2016.
|
| [11] |
陶真亚. 杂质离子对制备轻质碳酸钙的影响及其消除方法研究[D]. 杭州:浙江工业大学, 2013.
|
| [12] |
刘振法, 闫美芳, 张利辉, 等. 镁离子对碳酸钙晶型影响研究[J]. 水处理技术, 2011,37(10):60-62.
|
| [13] |
Dong W T, Liu R J, Zhao H, et al. Preparation and characterization of anhydrous magnesium carbonate under the present of potassium ions solution[J]. Powder Technology, 2019,360:741-746.
doi: 10.1016/j.powtec.2019.09.018
|
| [14] |
王明, 丁杨, 闫红旭, 等. 碳化法形貌可控制备碳酸钙的研究[J]. 无机盐工业, 2018,50(12):37-40.
|
| [15] |
Ihli J, Kim Y Y, Noel E H, et al. The effect of additives on amorph-ous calcium carbonate(ACC):Janus behavior in solution and the solid state[J]. Advanced Functional Materials, 2013,23(12):1575-1585.
doi: 10.1002/adfm.201201805
|
| [16] |
李梦维, 赵昌明, 程铁欣, 等. 十二烷基苯磺酸钠对碳酸钙结晶行为的影响[J]. 高等学校化学学报, 2018,39(11):2380-2385.
|
| [17] |
Zhang Z, Xie Y, Xu X, et al. Transformation of amorphous calcium carbonate into aragonite[J]. Journal of Crystal Growth, 2012,343(1):62-67.
doi: 10.1016/j.jcrysgro.2012.01.025
|
 ),Wang Xinyu1,2,3,Chen Tianyi1,2,3,Yang Xingshuo1,Zhao Yingying1,2,3,4(
),Wang Xinyu1,2,3,Chen Tianyi1,2,3,Yang Xingshuo1,Zhao Yingying1,2,3,4( ),Yuan Junsheng1,2,3,4(
),Yuan Junsheng1,2,3,4( )
)