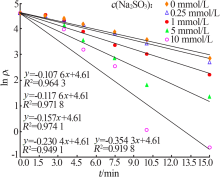
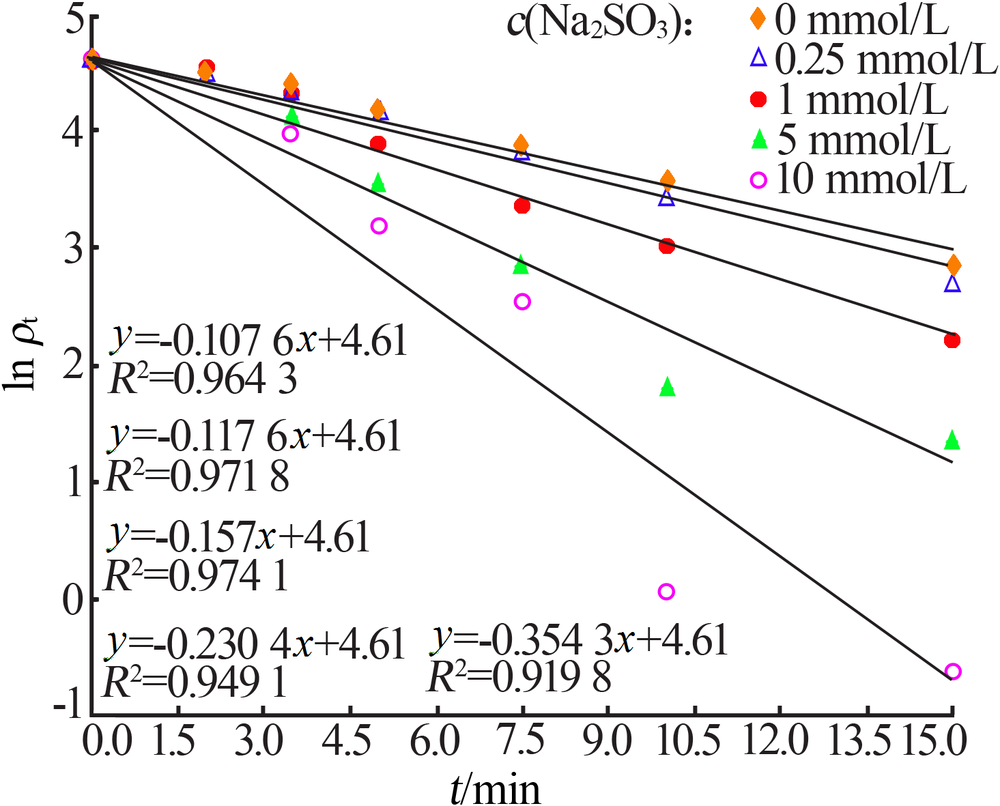
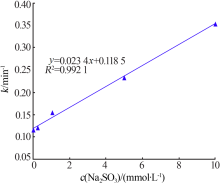
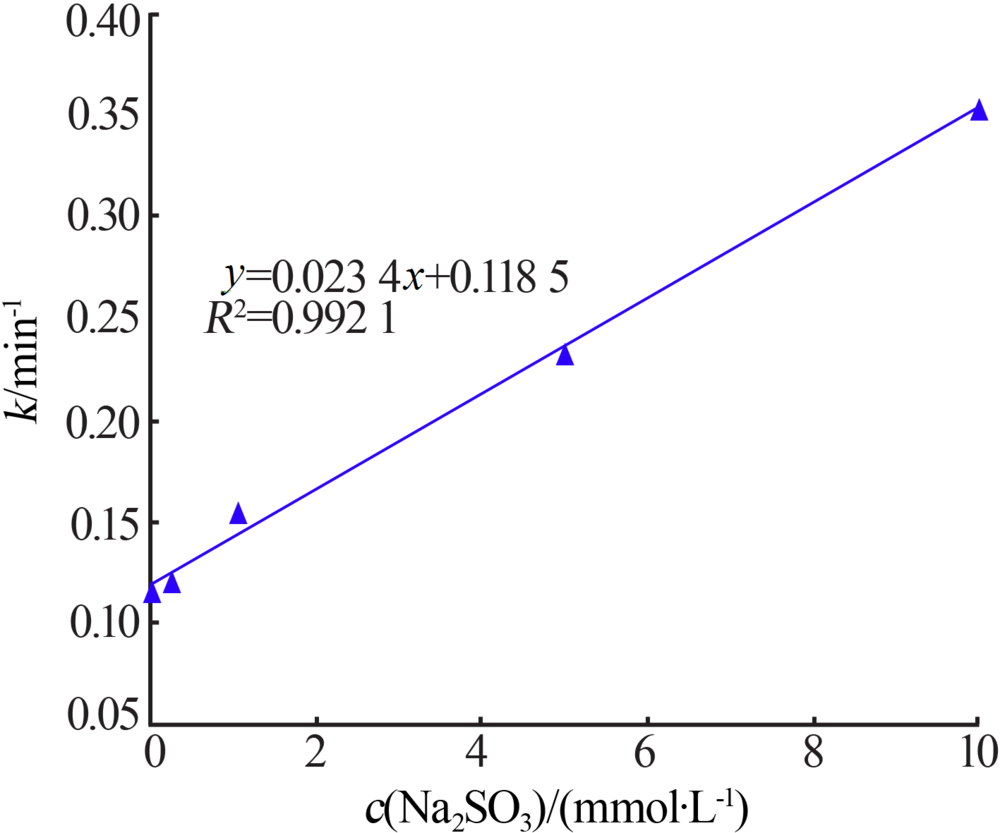
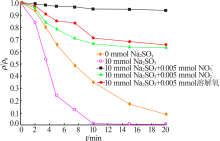
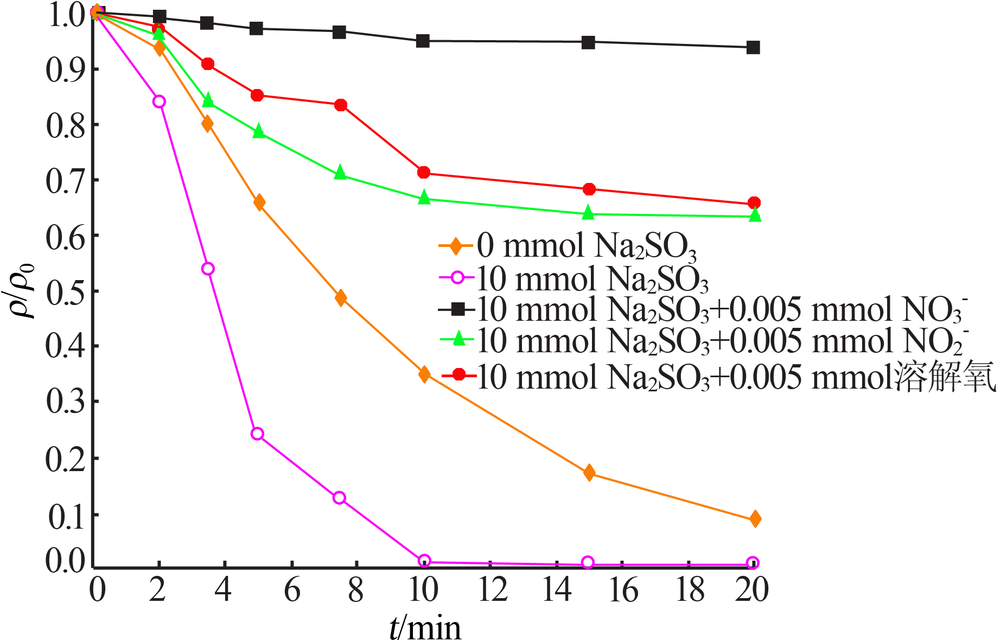
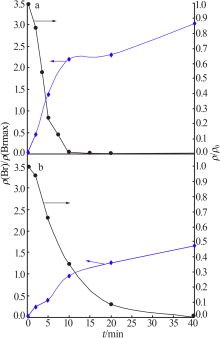
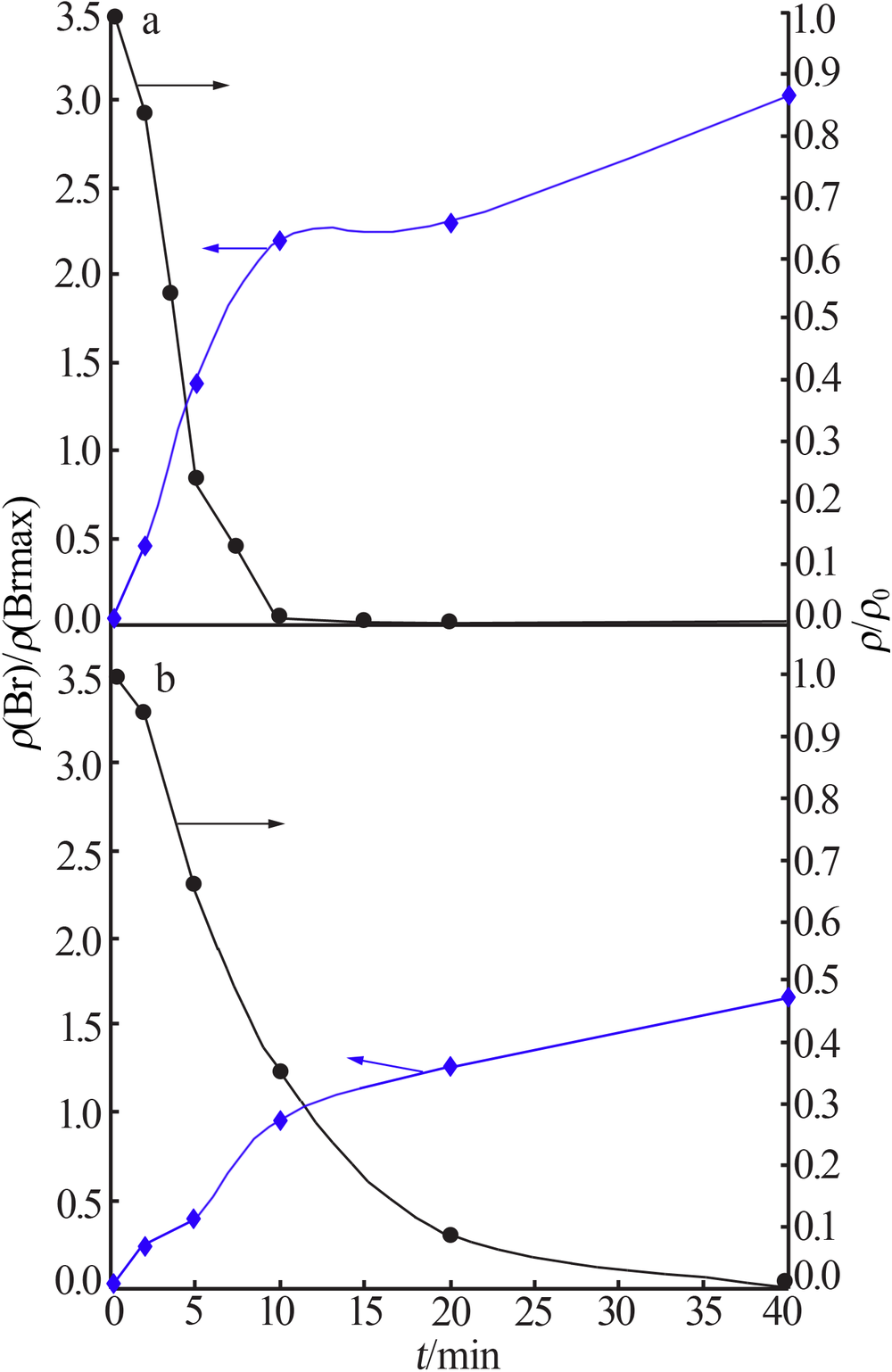
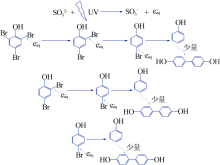
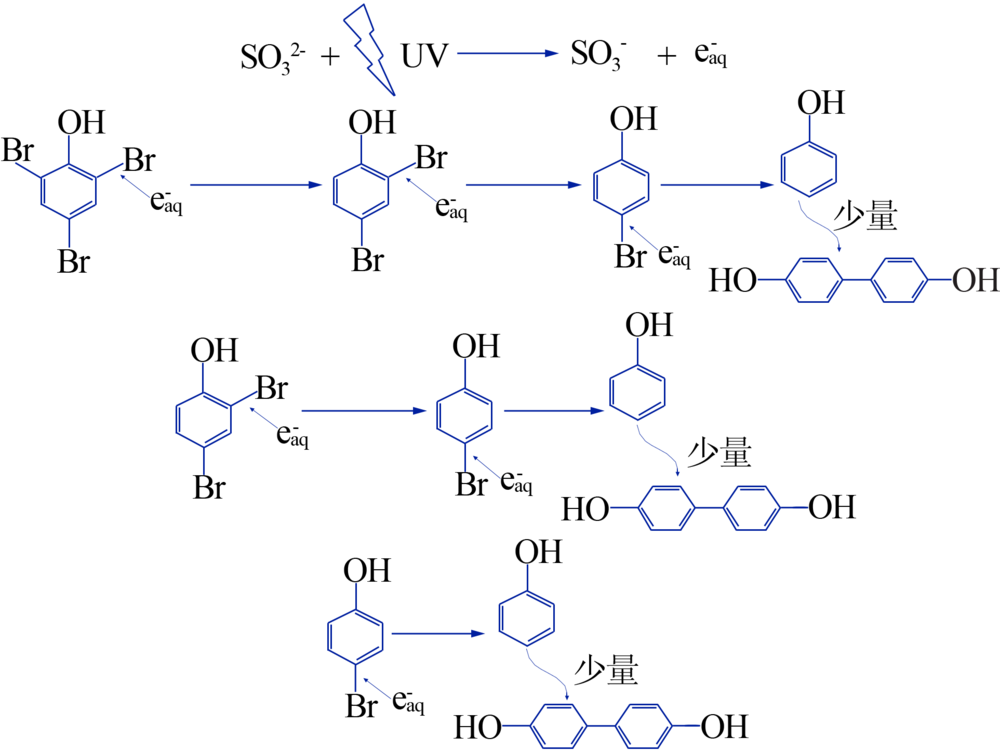
| [1] |
周楠楠, 张威, 赵金龙, 等. 废水深度处理的研究进展[J]. 无机盐工业, 2017,49(2):10-14.
|
| [2] |
化娜丽, 路帅, 赵东风, 等. 离子交换去除炼厂难降解废水中氯离子的静态实验研究[J]. 无机盐工业, 2015,47(11):66-69.
|
| [3] |
Zhang H, Shen Y, Liu W, et al. A review of sources,environmental occurrences and human exposure risks of hexachlorobutadiene and its association with some other chlorinated organics[J]. Environmental Pollution, 2019,253:831-840.
pmid: 31344544
|
| [4] |
张易旻, 陈铮铮, 陈昆柏, 等. 氯代有机物污染土壤高级化学氧化修复技术研究进展[J]. 环境化学, 2019,38(3):480-493.
|
| [5] |
Weng X, Meng Q, Liu J, et al. Catalytic oxidation of chlorinated organics over lanthanide perovskites:effects of phosphoric acid etching and water vapor on chlorine desorption behavior[J]. Environmental Science & Technology, 2019,53:884-893.
doi: 10.1021/acs.est.8b04582
pmid: 30472838
|
| [6] |
邓敏杰, 陈硕, 全燮, 等. 天然/模拟海水中2,4,6-三溴苯酚的光化学行为研究[J]. 环境科学学报, 2017,37(9):3427-3433.
|
| [7] |
项奇, 谢晟瑜, 张佳丽, 等. 氯酚类废水处理机理研究进展[J]. 工业水处理, 2017,37(11):5-10.
|
| [8] |
Chen C, Geng X, Huang W. Adsorption of 4-chlorophenol and aniline by nanosized activated carbons[J]. Chemical Engineering Journal, 2017,327:941-952.
|
| [9] |
Papazi A. Comparative biodegradation of all chlorinated phenols by the microalga scenedesmus obliquusthe biodegradation strategy of microalgae[J]. Journal of Biotechnology, 2019,296:61-68.
doi: 10.1016/j.jbiotec.2019.03.010
pmid: 30890327
|
| [10] |
Łecki T, Zarebska K, Sobczak K, et al. Photocatalytic degradation of 4-chlorophenol with the use of FTO/TiO2/SrTiO3 composite prepared by microwave-assisted hydrothermal method[J]. Applied Surface Science, 2019,470:991-1002.
|
| [11] |
Ji L, Cao X, Lu S, et al. Catalytic oxidation of PCDD/F on a V2O5WO3/TiO2 catalyst:effect of chlorinated benzenes and chlorinated phenols[J]. Journal of Hazardous Materials, 2018,342:220-230.
pmid: 28841469
|
| [12] |
Hinojosa-reyes M. Promotional effect of metal doping on nanostructured TiO2 during the photocatalytic degradation of 4-chlorophenol and naproxen sodium as pollutants[J]. Materials Science in Semiconductor Processing, 2019,100:130-139.
|
| [13] |
Xiao Q. Influence of UV lamp,sulfur(Ⅳ) concentration,and pH on bromate degradation in UV/sulfite systems:mechanisms and applications[J]. Water Research, 2017,111:288-296.
doi: 10.1016/j.watres.2017.01.018
pmid: 28104516
|
| [14] |
Li X, Ma J, Liu G, et al. Efficient reductive dechlorination of monochloroacetic acid by sulfite/UV process[J]. Environmental Science and Technology, 2012,46(13):7342-7349.
doi: 10.1021/es3008535
pmid: 22681542
|
| [15] |
Xie B, Li X, Huang X, et al. Enhanced debromination of 4-bromophenol by the UV/sulfite process:Efficiency and mechanism[J]. Journal of Environmental Sciences, 2017,54:231-238.
|
| [16] |
Liu X, Yoon S, Batchelor B, et al. Degradation of vinyl chloride (VC) by the sulfite/UV advanced reduction process(ARP):Effects of process variables and a kinetic model[J]. Science of the Total Environment, 2013,454:578-583.
|
| [17] |
Song Z. Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system[J]. Journal of Hazardous Materials, 2013,262:332-338.
pmid: 24056245
|
| [18] |
伏芝萱, 郭迎庆, 楚文海. 紫外/亚硫酸钠还原降解三氯乙酰胺的效能[J]. 环境科学, 2019,40(5):2271-2277.
|
| [19] |
Herrman H. On the photolysis of simple anions and neutral molecules as sources of O-/OH,SOx- and Cl in aqueous solution[J]. Physical Chemistry Chemical Physics, 2007,9(30):3935-3964.
pmid: 17646883
|
| [20] |
Sauer M C, Shkrob I A, Lian R, et al. Electron photodetachment from aqueous anions.II.Ionic strength effect on geminate recombination dynamics and quantum yield for hydrated electron[J]. Journal of Physical Chemistry A, 2004,108:10414-10425.
|
| [21] |
Qu Y, Zhang C. Photo-reductive defluorination of perfluorooctanoic acid in water[J]. Water Research, 2010,44(9):2939-2947.
doi: 10.1016/j.watres.2010.02.019
pmid: 20227745
|
 ),Cheng Ting1,Chen Chen3(
),Cheng Ting1,Chen Chen3( ),Wang Jinnan2,4,Chen Gang3,Deng Qin3,Wang Lei3
),Wang Jinnan2,4,Chen Gang3,Deng Qin3,Wang Lei3