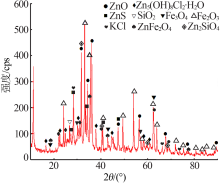
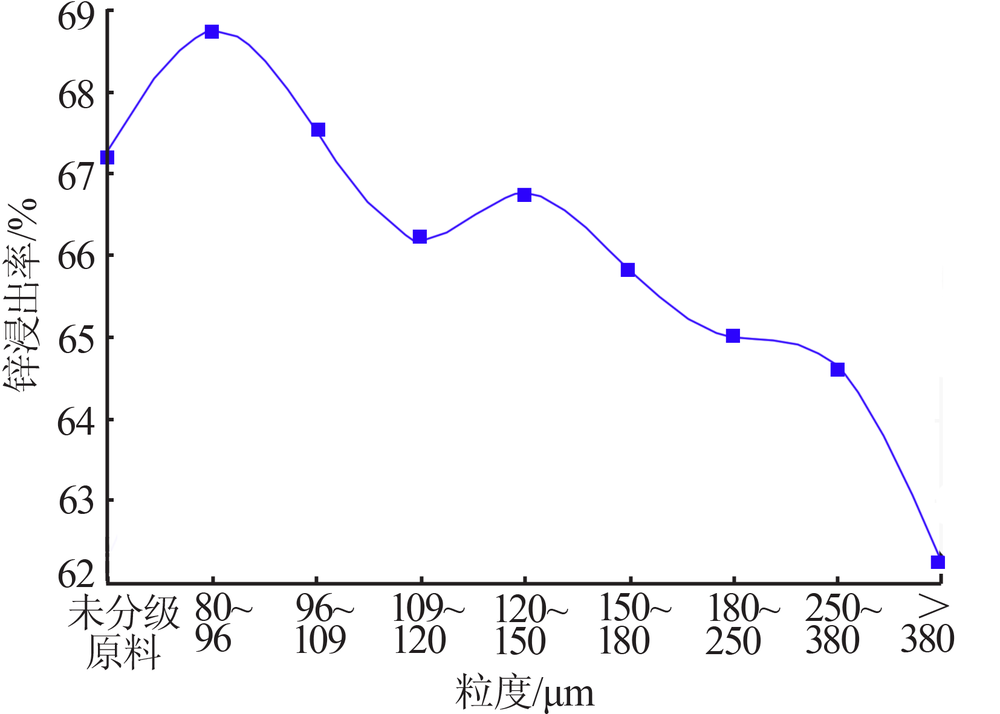
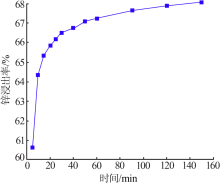
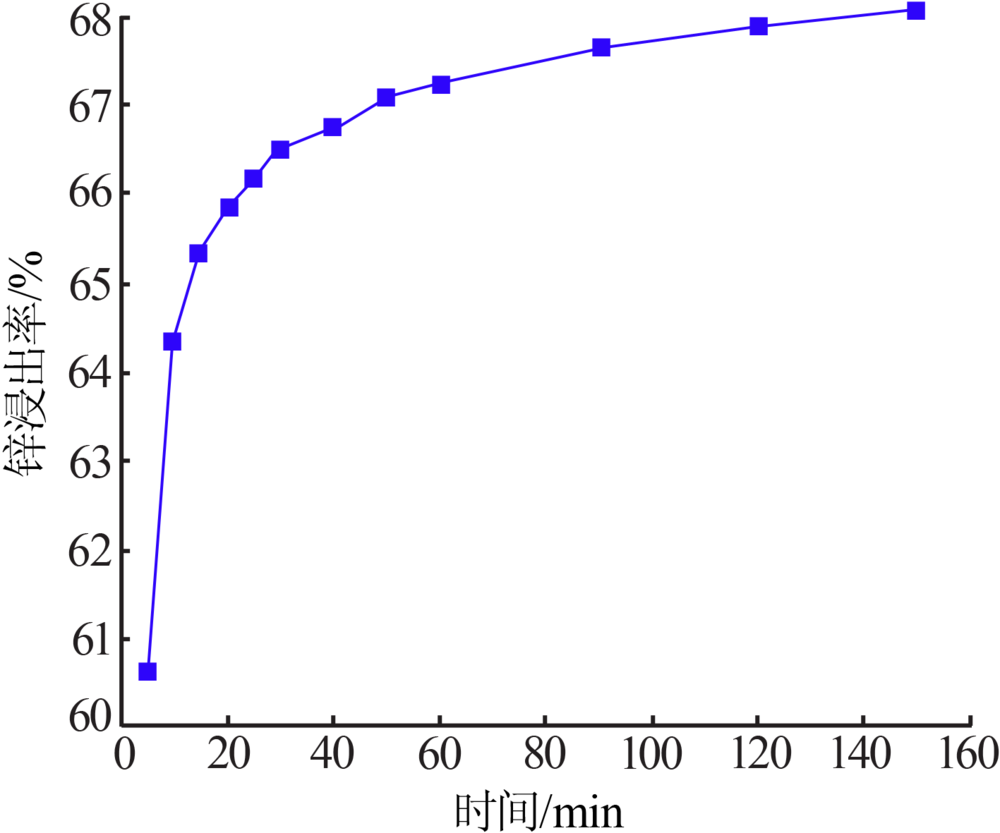
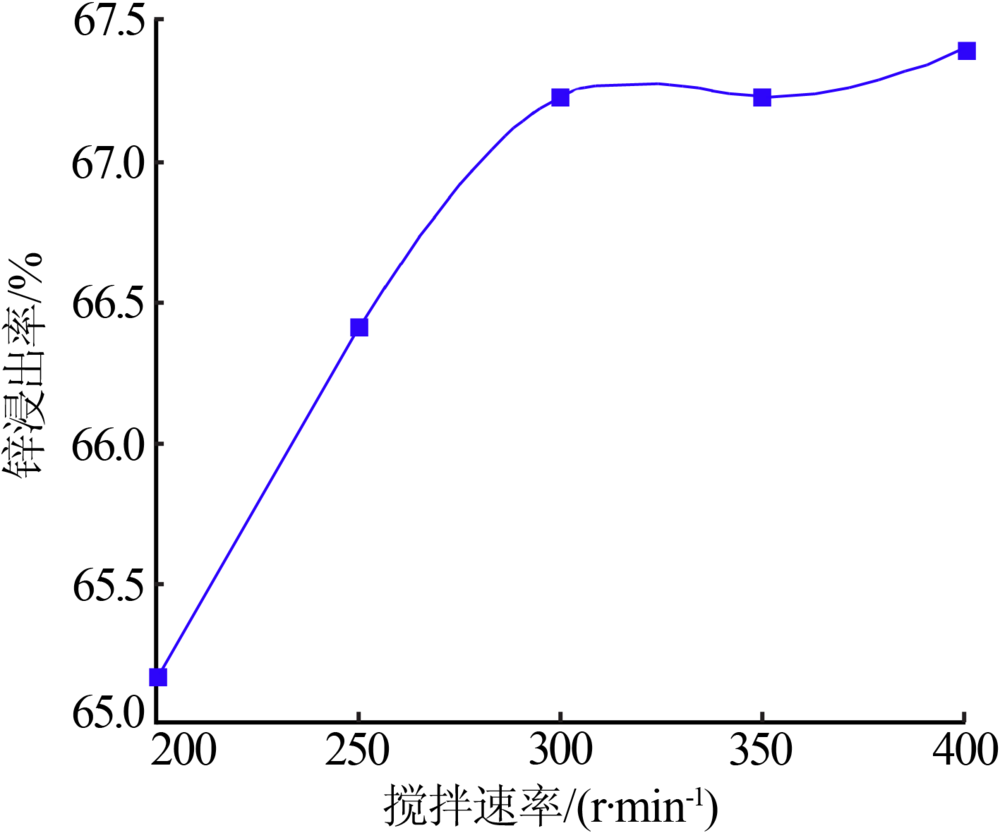
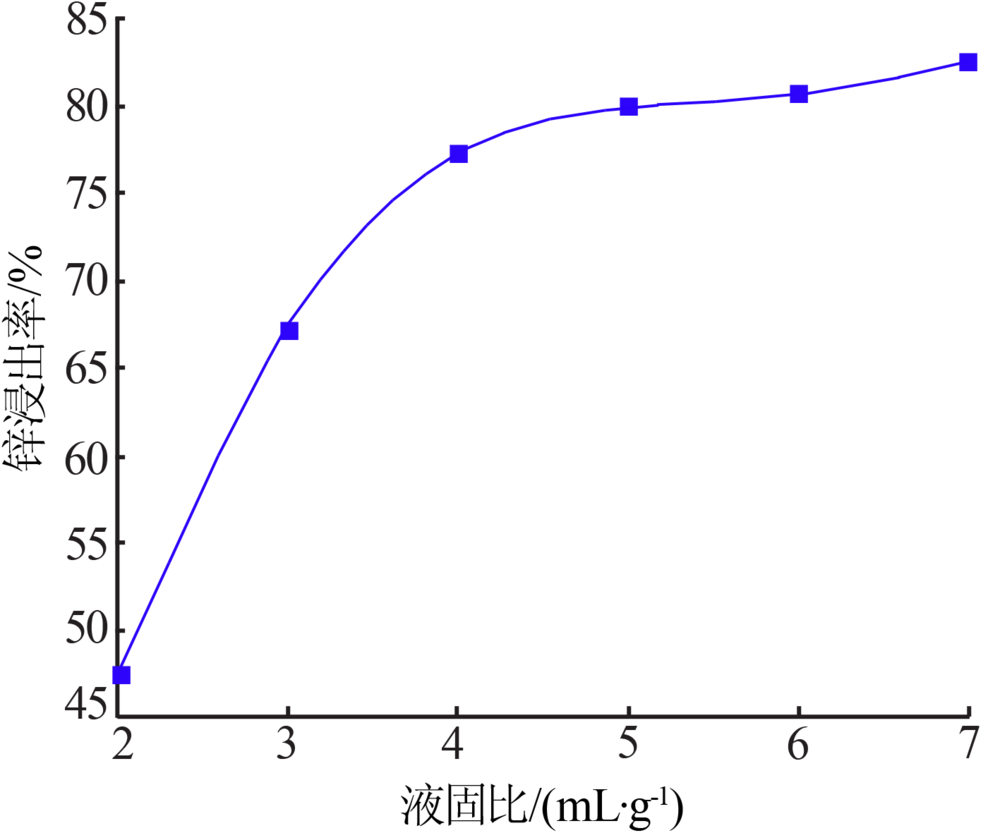
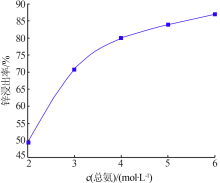
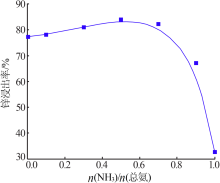
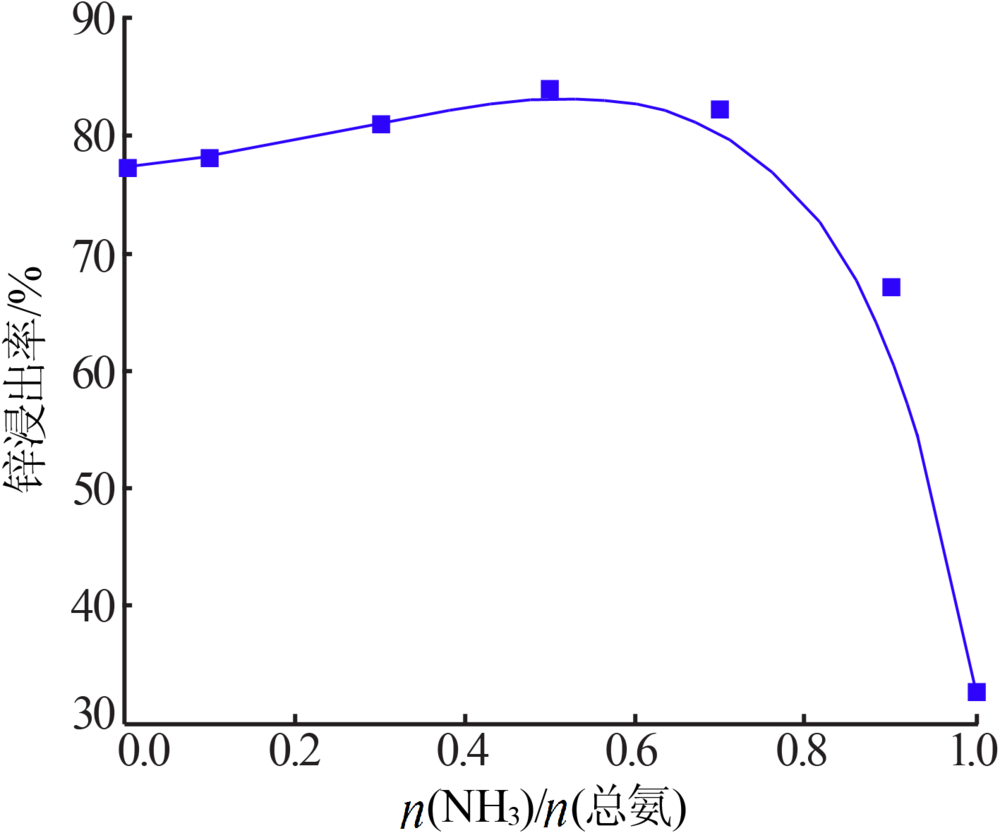
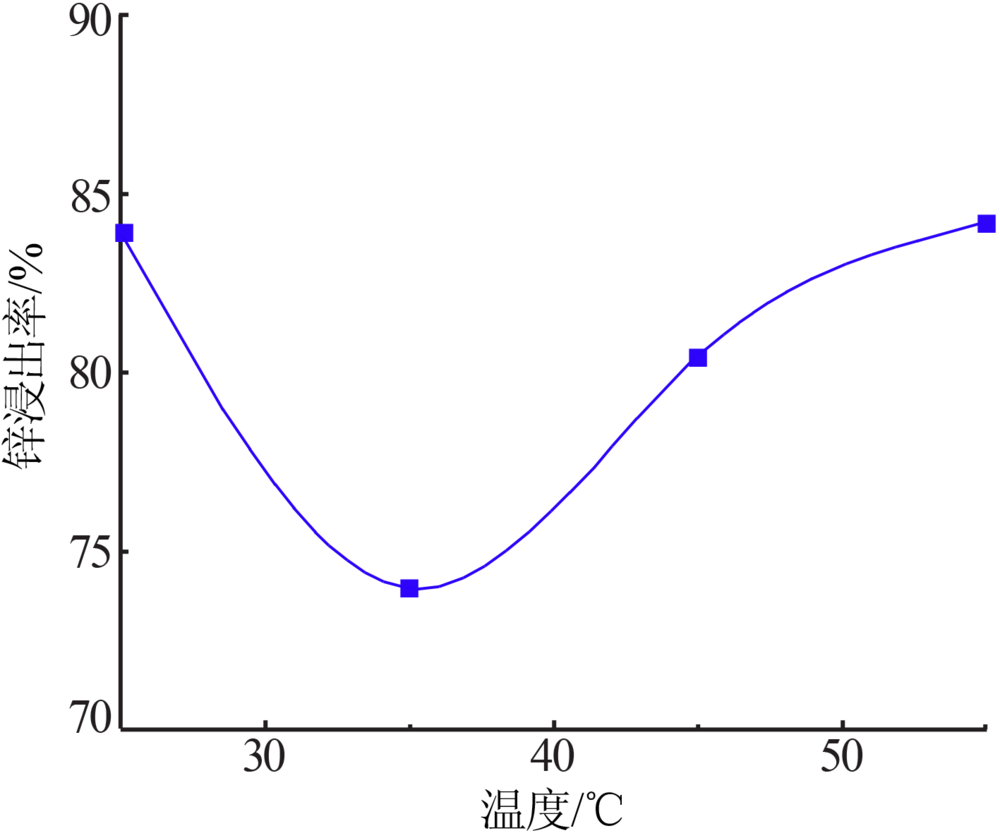
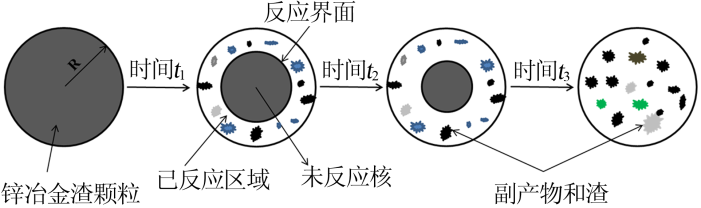
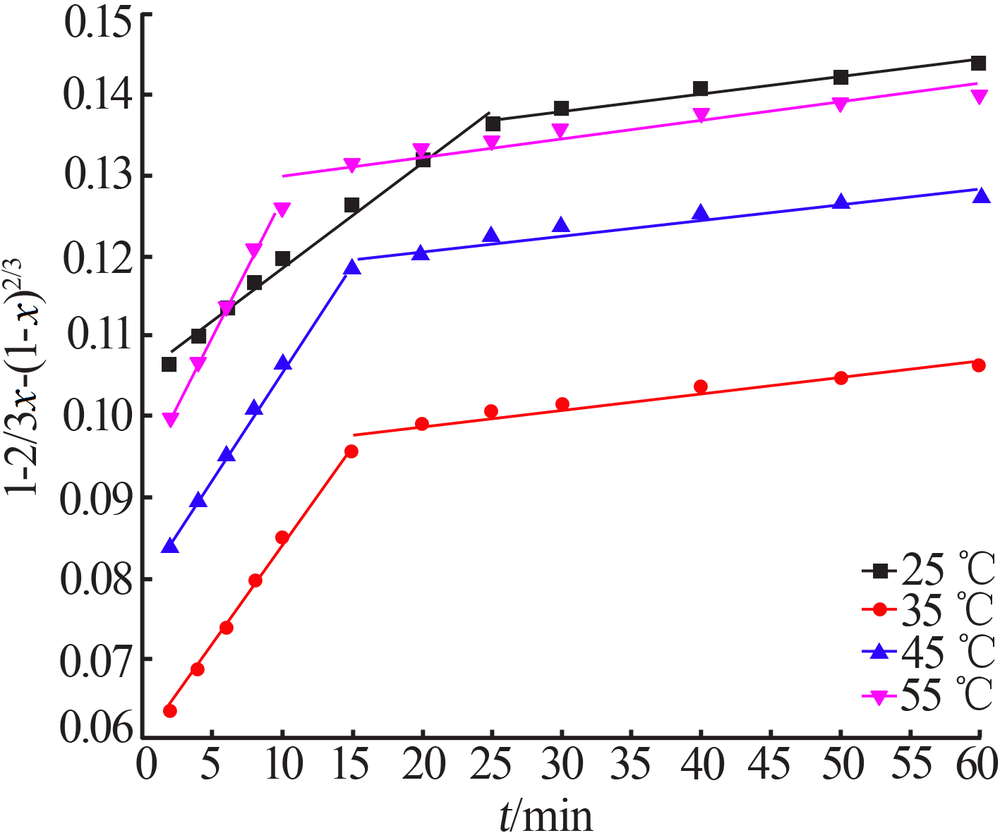
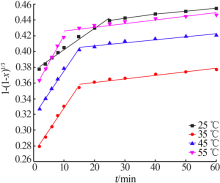
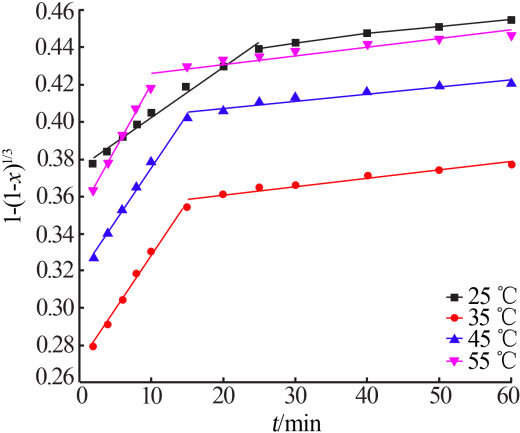
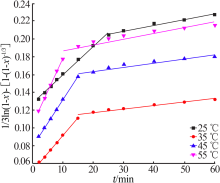
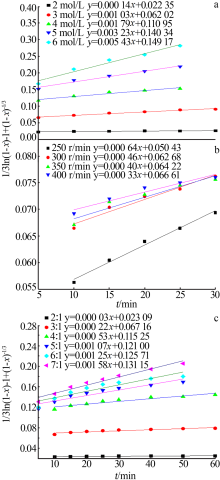
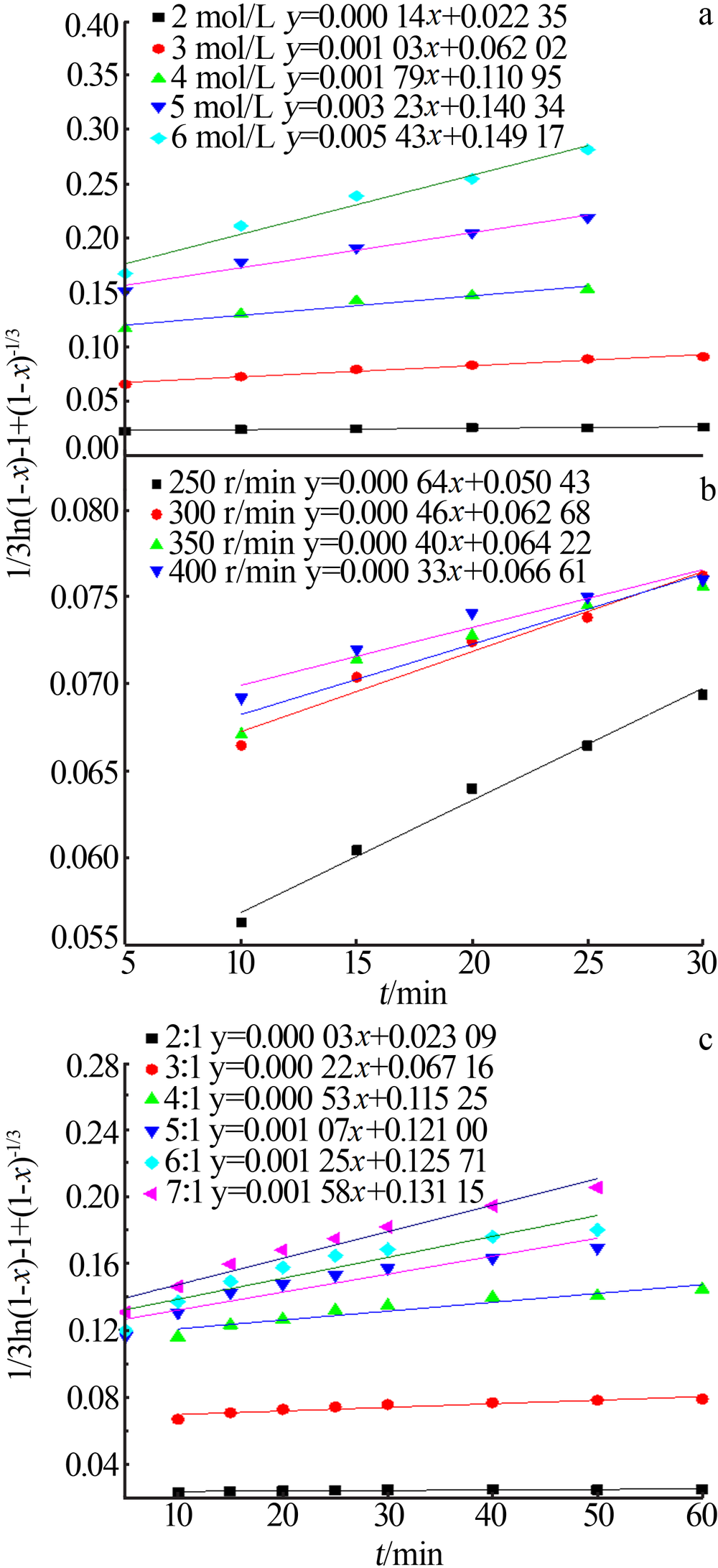
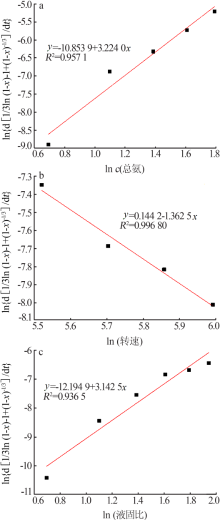
Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (11): 69-74.doi: 10.11962/1006-4990.2019-0643
• Environment·Health·Safety • Previous Articles Next Articles
Study on leaching process and kinetics of zinc extraction from metallurgical slag and dust with ammonia
Ma Aiyuan( ),Zheng Xuemei(
),Zheng Xuemei( ),Li Song
),Li Song
- School of Chemistry and Materials Engineering,Liupanshui Normal University,Liupanshui 553004,China
-
Received:2020-05-17Online:2020-11-10Published:2020-12-01 -
Contact:Zheng Xuemei E-mail:may_kmust11@163.com;zxm_lpssy19@163.com
CLC Number:
Cite this article
Ma Aiyuan,Zheng Xuemei,Li Song. Study on leaching process and kinetics of zinc extraction from metallurgical slag and dust with ammonia[J]. Inorganic Chemicals Industry, 2020, 52(11): 69-74.
share this article
| [1] | 刘红召, 杨卉芃, 冯安生. 全球锌矿资源分布及开发利用[J].矿产保护与利用, 2017(1):113-118. |
| [2] | 孙传尧, 宋振国, 朱阳戈, 等. 中国铜铝铅锌矿产资源开发利用现状及安全供应战略研究[J].中国工程科学, 2019(1):133-139. |
| [3] | 宋凯伟, 李佳磊, 蔡锦鹏, 等. 典型氧化铜铅锌矿物浮选的表面硫化研究进展[J]. 化工进展, 2018,37(9):341-351. |
| [4] | 周英伟, 高波. 热镀锌渣回收利用工艺研究进展[J]. 表面技术, 2017,46(10):91-98. |
| [5] | Tang Lei, Tang Chaobo, Xiao Jin, et al. A cleaner process for lead re-covery from lead-containing hazardous solid waste and zinc leaching residue via reducing-matting smelting[J]. Journal of Cleaner Produc-tion, 2019,241.Doi: 10.1016/j.jclepro.2019.118328 . |
| [6] | 罗龙海, 闫艳梅, 袁建伟, 等. 废旧锌锰电池中锰和锌在硫酸/草酸溶液中的浸出行为[J]. 湿法冶金, 2019,38(6):469-472. |
| [7] | Pan D A, Lia L L, Tian X, et al. A review on lead slag generation,characteristics,and utilization[J]. Resources,Conservation & Re-cycling, 2019,146:140-155. |
| [8] | 田伟军. 用氨配合法从含锌废催化剂中回收锌[J]. 无机盐工业, 2016,48(2):55-58. |
| [9] | Meng L, Zhong Y W, Guo L, et al. Recovery of Cu and Zn from waste printed circuit boards using super-gravity separation[J]. Waste Ma-nagement, 2018,78:559-565. |
| [10] | 牛永胜, 程亮, 李银丽, 等. 浸锌渣-高杂氧化锌铅锌回收工艺研究[J]. 甘肃冶金, 2019,47(4):16-18. |
| [11] | 王冬斌, 梁精龙, 李慧, 等. 冶金含锌粉尘中回收氧化锌的工艺综述[J]. 中国有色冶金, 2018,47(6):46-50. |
| [12] | 雷华志, 杨光灿, 李雨耕, 等. 从锌渣中直接热力回收金属锌的工艺研究[J]. 云南冶金, 2018,47(4):63-66. |
| [13] | 王超, 郭宇峰, 杨凌志, 等. 含锌渣尘中有价金属回收利用现状与研究进展[J]. 金属矿山, 2019,48(3):27-35. |
| [14] |
Sethurajan M, Huguenot D, Jain R, et al. Leaching and selective zinc recovery from acidic leachates of zinc metallurgical leach re-sidues[J]. Journal of Hazardous Materials, 2016,324:71-82.
doi: 10.1016/j.jhazmat.2016.01.028 pmid: 26832075 |
| [15] | Fattahi A, Rashchi F, Abkhoshk E. Reductive leaching of zinc,co-balt and manganese from zinc plant residue[J]. Hydrometallurgy, 2016,161:185-192. |
| [16] | Ashtari P, Pourghahramani P. Selective mechanochemical alkaline leaching of zinc from zinc plant residue[J]. Hydrometallurgy, 2015,156:165-172. |
| [17] | Steer J M, Griffiths A J. Investigation of carboxylic acids and non-aqueous solvents for the selective leaching of zinc from blast furnace dust slurry[J]. Hydrometallurgy, 2013,140:34-41. |
| [18] | 刘智勇. 氧化锌矿物在氨-铵盐-水体系中的浸出机理[D]. 长沙:中南大学, 2012. |
| [19] | Rao S, Yang T Z, Zhang D C, et al. Leaching of low grade zinc oxide ores in NH4Cl-NH3,solutions with nitrilotriacetic acid as complexing agents[J]. Hydrometallurgy, 2015,158:101-106. |
| [20] | Popescu I A, Varga T, Egedy A, et al. Kinetic models based on an-alysis of the dissolution of copper,zinc and brass from WEEE in a sodium persulfate environment[J]. Computers & Chemical Engin-eering, 2015,83:214-220. |
| [1] | Hou Lishuang,Gu Shouyu,Hou Cuihong,Wang Haobin,Li Luyi. Optimization of preparation process of sodium pyrophosphate chelates zinc by response surface analysis [J]. Inorganic Chemicals Industry, 2020, 52(7): 30-35. |
| [2] | Zhou Jian,Tang Hongbo. Preparation and photocatalytic properties of ZnWO4/Ag3PO4 composite photocatalyst [J]. Inorganic Chemicals Industry, 2020, 52(6): 101-104. |
| [3] | Ou Xiaoju,Chai Qi,Li Zhengke,Wang Weiyan,Yang Yunquan. Preparation of battery grade iron phosphate from phosphating slag [J]. Inorganic Chemicals Industry, 2020, 52(10): 135-139. |
| [4] | Hu Min,Guo Jia,Wu Huadong,Zhang Linfeng. Photocatalytic oxidation of sodium sulfite in desulfurization wastewater by N-Zn/TiO2 [J]. Inorganic Chemicals Industry, 2020, 52(10): 151-156. |
| [5] | Su Xiaoli,Qin Fengting,Cai Tiancong,Ma Chunyu,Tang Changqing. Preparation of nano-sized zinc oxide by thermal decomposition with different carbonate precipitates [J]. Inorganic Chemicals Industry, 2019, 51(9): 36-39. |
| [6] | Kuang Yi,Li Jun,Jin Yang,Chen Ming,Zuo Longtao. Synthesis of nano-sized zinc oxide in liquid phase and its photocatalytic properties [J]. Inorganic Chemicals Industry, 2019, 51(9): 40-44. |
| [7] | Chen Xinyi,Huang Yunlong,Yuan Aiqun,Huang Zengwei,Wei Dongping,Ma Shaomei. Controlled-synthesis of nanometer zinc phosphate [J]. Inorganic Chemicals Industry, 2019, 51(8): 20-24. |
| [8] | Feng Junli,Yang Junfan,Wu Jingwu,Li Xu,Zhang Qingjian. Determination of mercury in crude zinc oxide by DMA-80 mercury analyzer [J]. Inorganic Chemicals Industry, 2019, 51(8): 73-75. |
| [9] | Li Jiekang,Xie Jixing. Mechano-chemical preparation of zinc stannate and its flame-retardant application in PVC [J]. Inorganic Chemicals Industry, 2019, 51(5): 33-37. |
| [10] | Liang Wen,Wang Xinlong,Chen Jianjun,Xie Wenji,Leng Xinke,Ling Haohan. Study on law of chelated Zn by water?鄄soluble ammonium polyphosphate [J]. Inorganic Chemicals Industry, 2019, 51(11): 20-22. |
| [11] | XU Su-Peng, TANG Chang-Qing, LI Xiao-Le, SU Xiao-Li, XU Yin-Xia. Study on process conditions of low cost preparation of nanometer zinc oxide [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(9): 68-. |
| [12] | XIANG Le-Kai, LI Feng, ZHAO Ning, WANG Feng, XIAO Fu-Kui, WANG Jun-Wei. Study on preparation of calcium carbonate by carbon dioxide bubbling carbonation [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(8): 46-. |
| [13] | XU Jian-Zhong, HE Li-Qian, FENG Gang, XIE Ji-Xing. Preparation of amorphous zinc stannate coated clay and its flame retardant application [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(3): 17-. |
| [14] | TIAN Wei-Jun. Recovery of zinc from zinc-containing catalyst by ammonia complex method [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(2): 49-. |
| [15] | YANG Jian-Wen, YOU Hai-Ping, DENG Xing-Shen, PAN Xiao-Jin, YANG Gui-Jun, ZHANG Ling-Zhi. Preparation and electrochemical performances of chiral porous sodium zinc phosphate as anode material [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(2): 75-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|