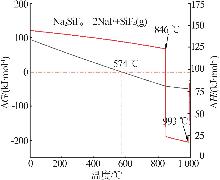
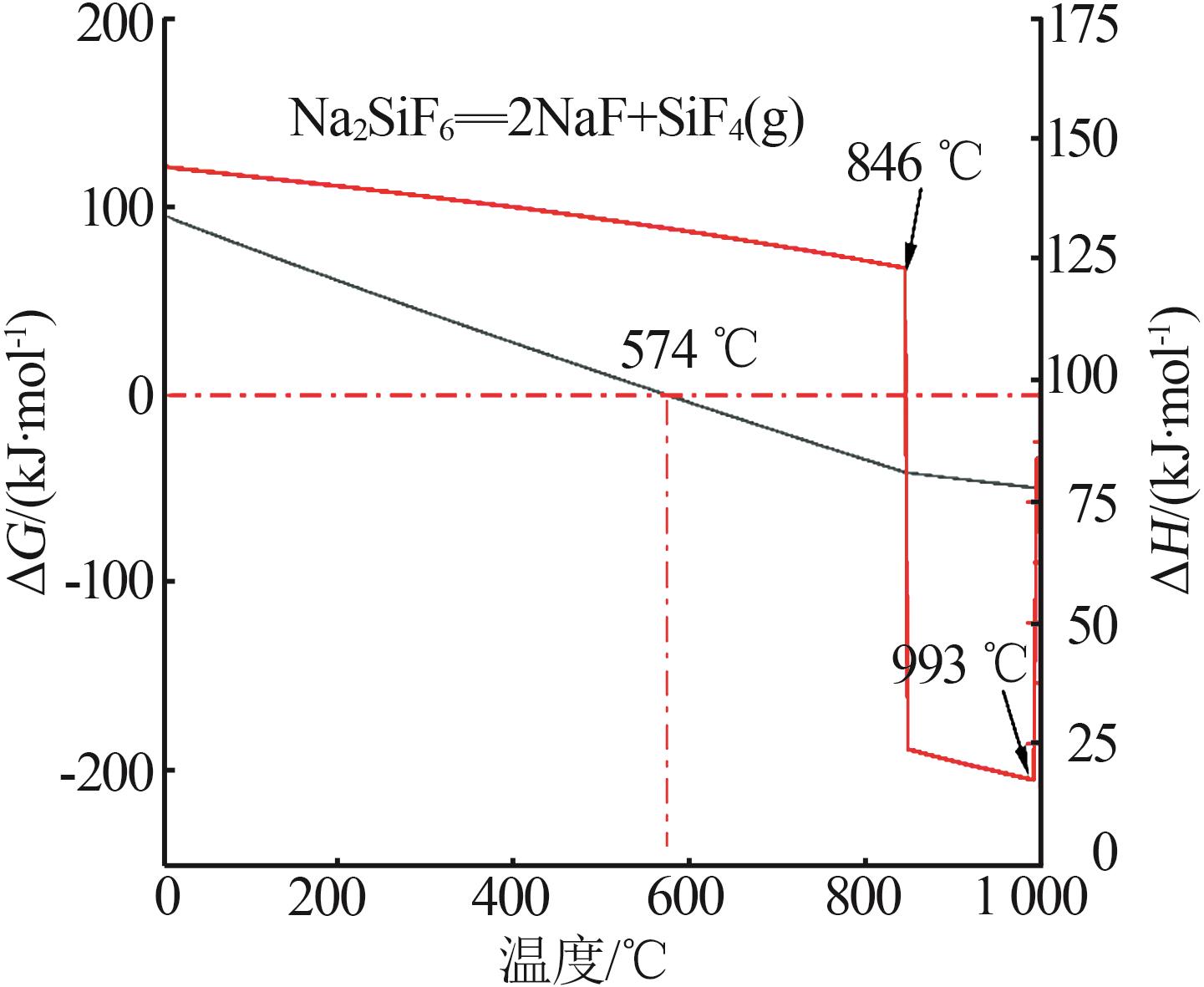
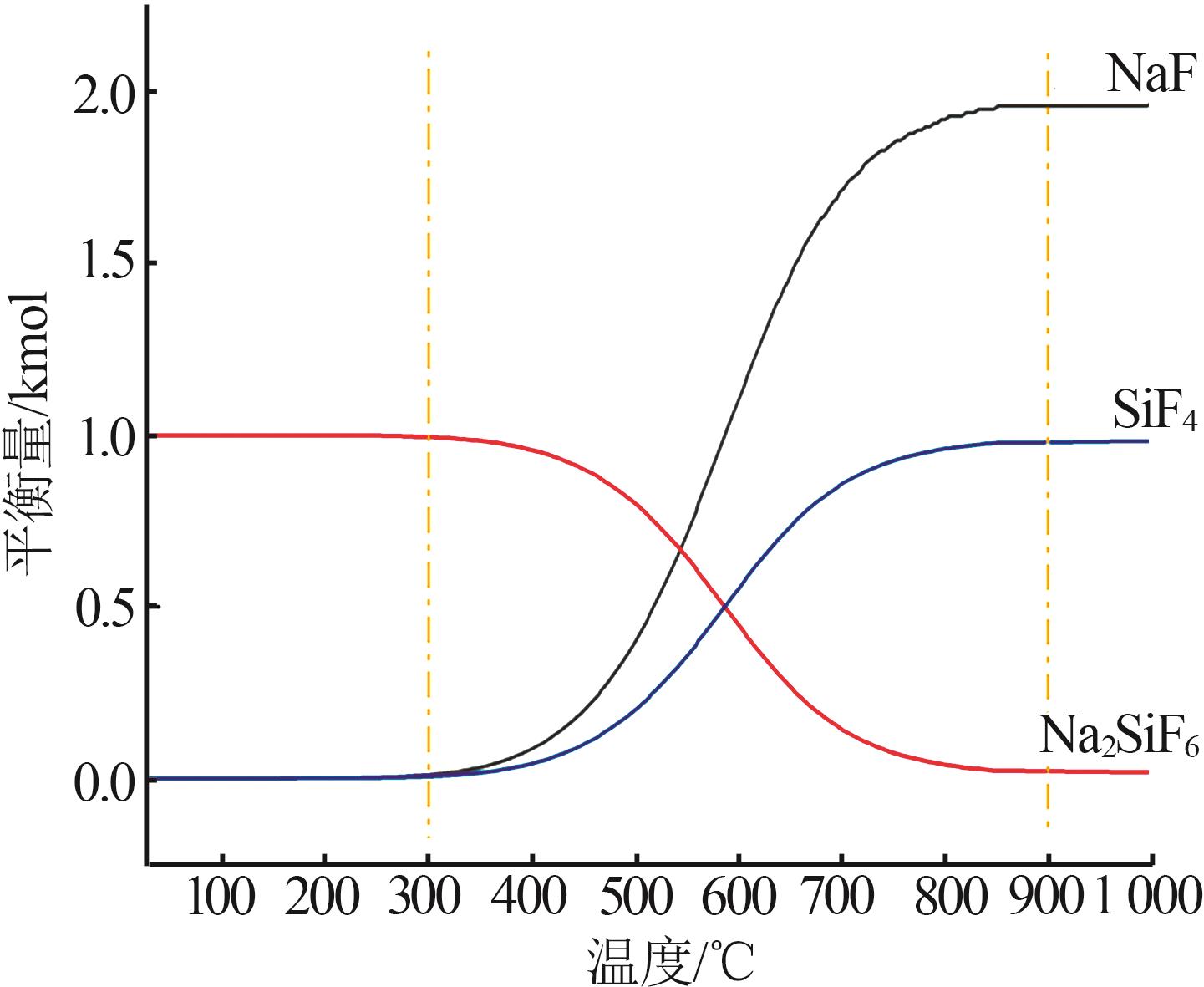
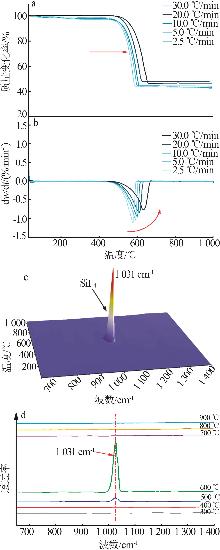
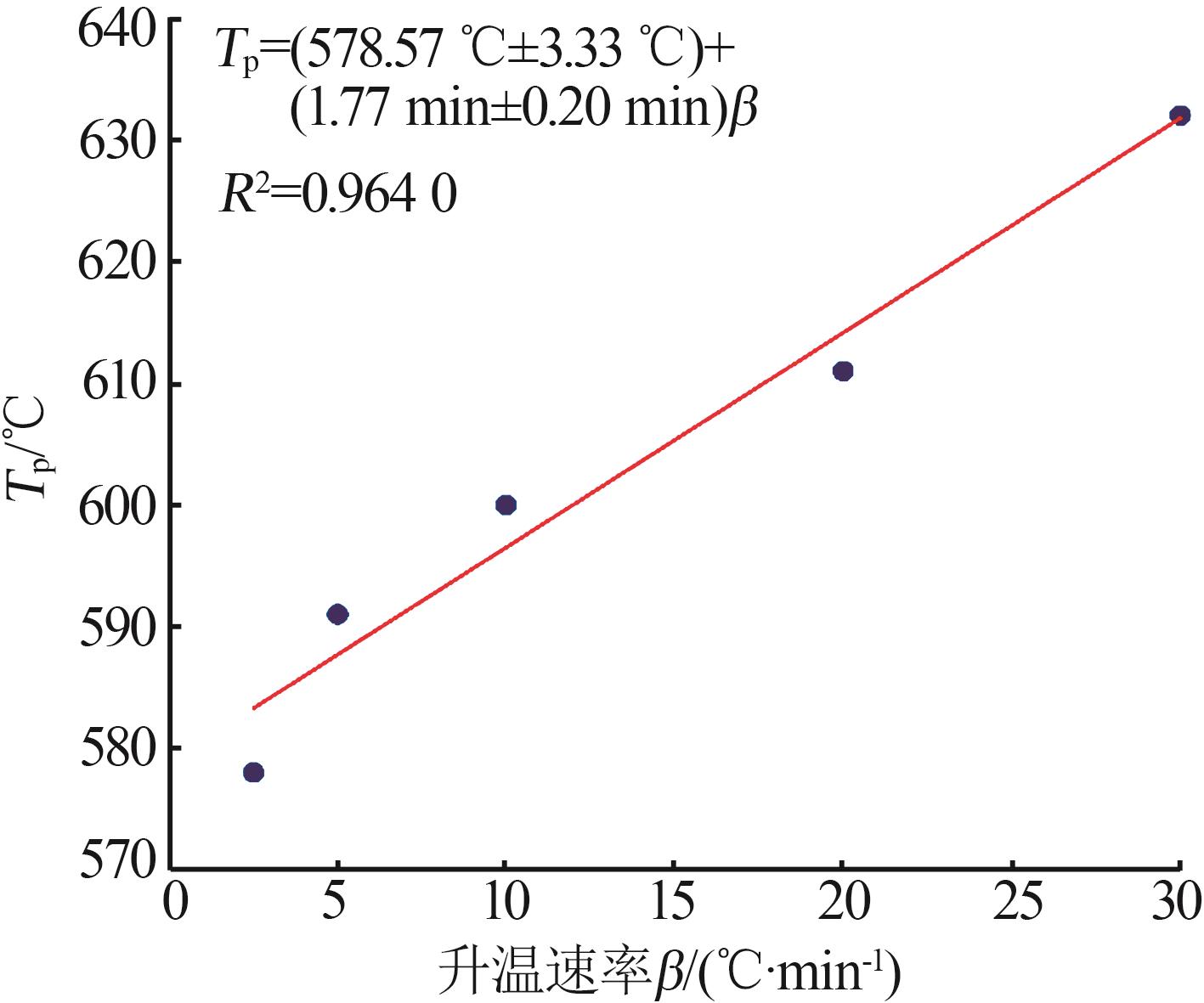
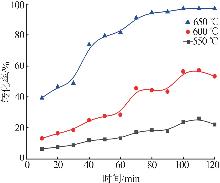
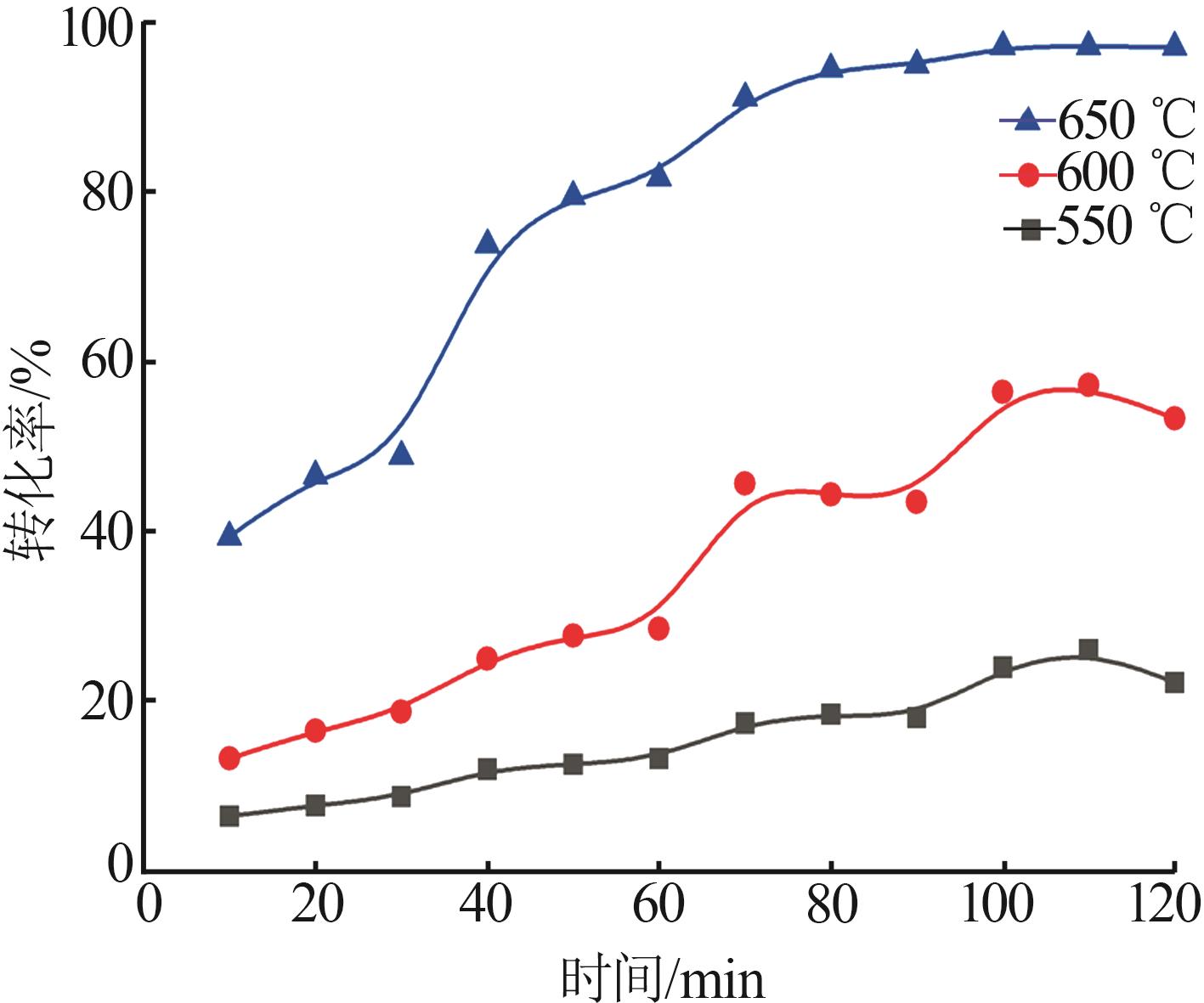
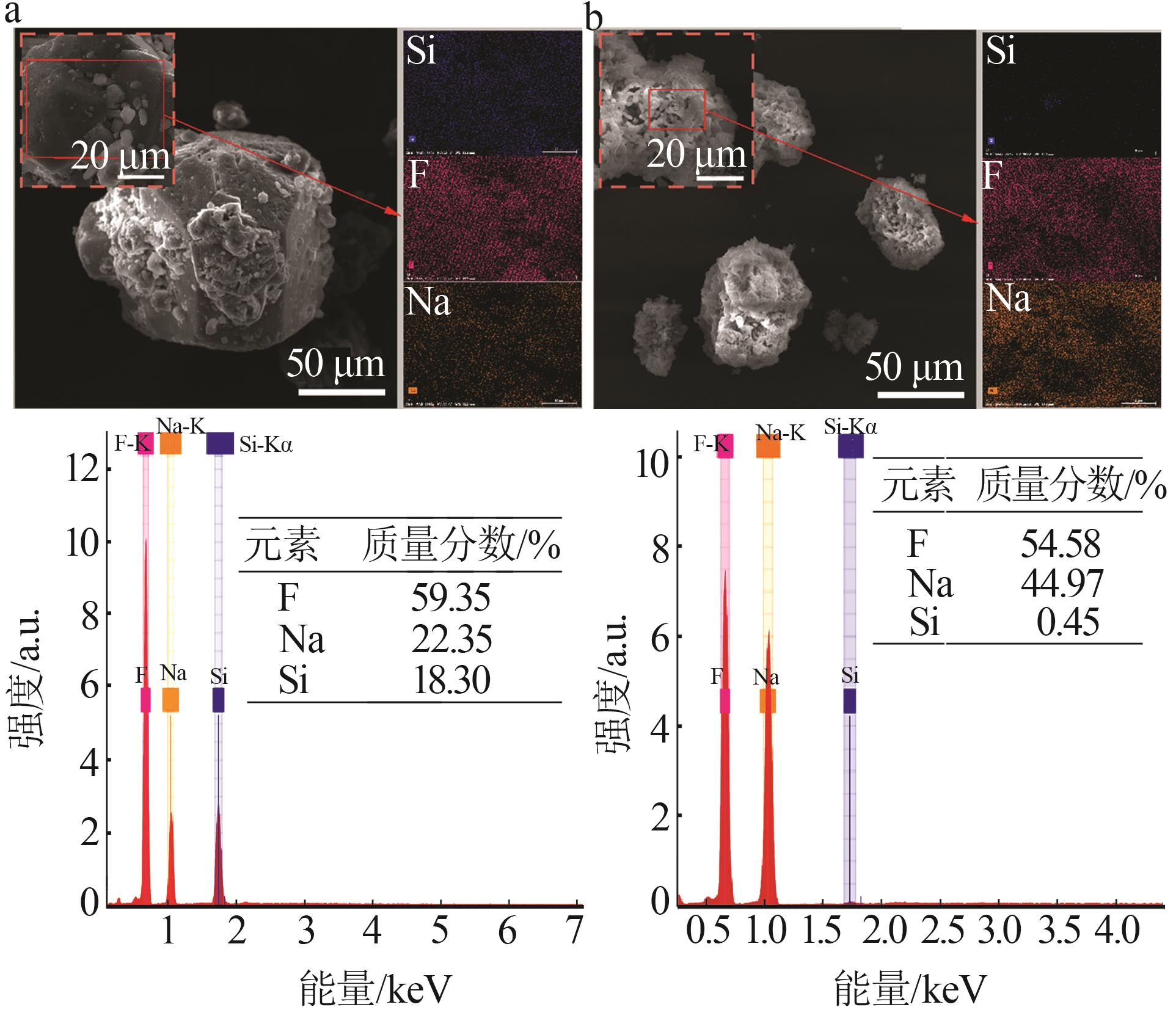
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (8): 28-34.doi: 10.19964/j.issn.1006-4990.2024-0462
• Research & Development • Previous Articles Next Articles
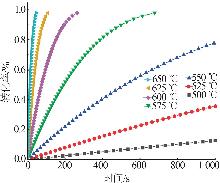
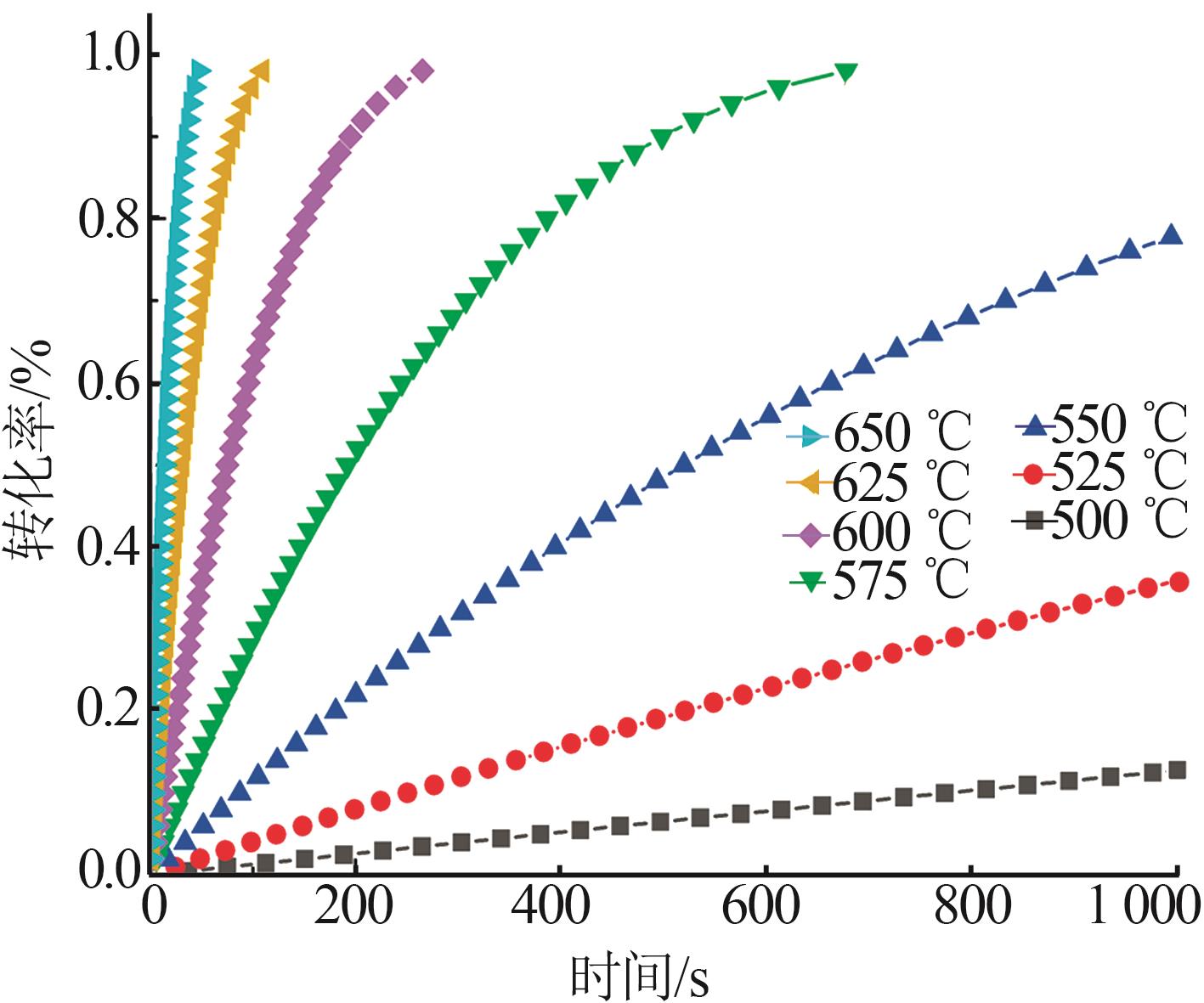
Study on thermal decomposition kinetics of sodium fluosilicate
SHOU Zhixin( ), XU Dehua(
), XU Dehua( ), LIU Yujia, YANG Wengong, WANG Xinlong
), LIU Yujia, YANG Wengong, WANG Xinlong
- Ministry of Education Research Center for Comprehensive Utilization and Clean Processing Engineering of Phosphorus Resources,School of Chemical Engineering,Sichuan University,Chengdu 610065,China
-
Received:2024-08-20Online:2025-08-10Published:2024-09-09 -
Contact:XU Dehua E-mail:953560434@qq.com;dhxu@scu.edu.cn
CLC Number:
Cite this article
SHOU Zhixin, XU Dehua, LIU Yujia, YANG Wengong, WANG Xinlong. Study on thermal decomposition kinetics of sodium fluosilicate[J]. Inorganic Chemicals Industry, 2025, 57(8): 28-34.
share this article
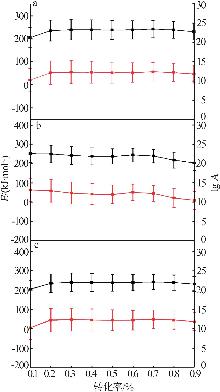
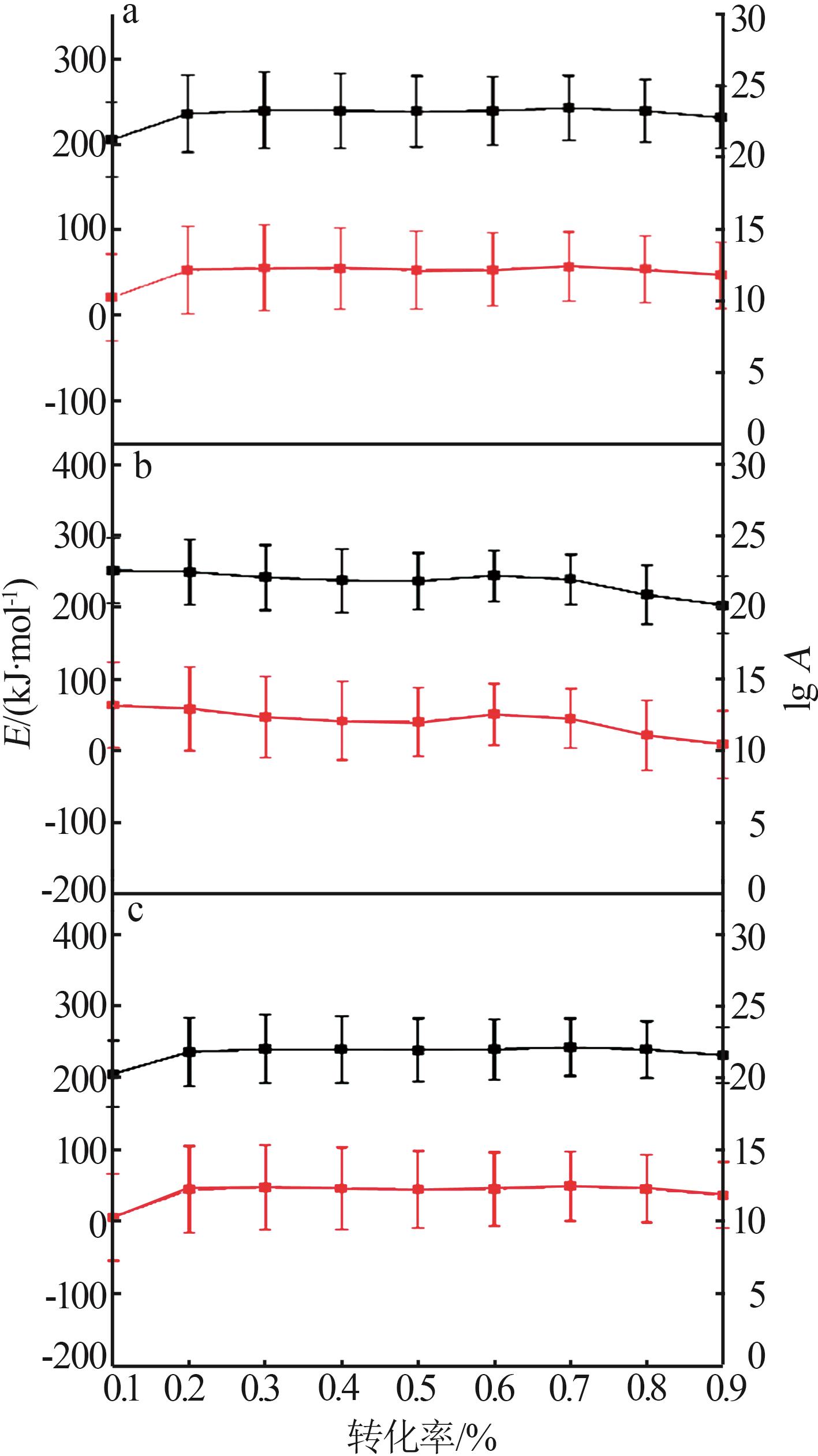
Table 3
Solving results of 16 mechanism functions"
机理 模型 | 微分公式 | 数值 | R2 | |
|---|---|---|---|---|
| E/(kJ·mol-1) | lg A | |||
| F1 | 1-α | 242.66 | 12.55 | 0.997 94 |
| F2 | (1-α)2 | 299.32 | 16.36 | 0.997 88 |
| Fn | (1-α) n | 262.58 | 13.90 | 0.998 11 |
| R2 | 2(1-α)1/2 | 235.36 | 11.68 | 0.997 20 |
| R3 | 3(1-α)2/3 | 231.32 | 11.28 | 0.997 52 |
| D1 | 1/2α | 289.02 | 14.98 | 0.998 85 |
| D2 | [-ln(1-α)]-1 | 314.33 | 16.40 | 0.998 88 |
| D3 | 3/2(1-α)2/3[1-(1-α)1/3]-1 | 346.70 | 17.92 | 0.998 70 |
| D4 | 3/2[(1-α)1/3-1]-1 | 324.59 | 16.44 | 0.998 84 |
| B1 | α(1-α) | 328.51 | 19.23 | 0.985 94 |
| Bna | αn (1-α)m | 262.23 | 13.89 | 0.998 09 |
| C1 | (1-α)(1+Kcatα) | 229.11 | 11.57 | 0.997 37 |
| Cn | (1-α) n (1+Kcatα) | 259.20 | 13.63 | 0.997 92 |
| A2 | 2(1-α)[-ln(1-α)]1/2 | 210.16 | 10.55 | 0.993 49 |
| A3 | 3(1-α)[-ln(1-α)]2/3 | 218.85 | 11.13 | 0.990 47 |
| An | n(1-α)[-ln(1-α)](n-1)/n | 307.17 | 16.57 | 0.998 62 |
| [1] | 陈军元,刘艳飞,颜玲亚,等.石墨、萤石等战略非金属矿产发展趋势研究[J].地球学报,2021,42(2):287-296. |
| CHEN Junyuan, LIU Yanfei, YAN Lingya,et al.Research on development trend of strategic nonmetallic minerals such as graphite and fluorite[J].Acta Geoscientica Sinica,2021,42(2):287-296. | |
| [2] | 杨勇,陈海洋,李煜坤,等.无水氟化氢和氟化铝工艺研发及工业应用进展[J].无机盐工业,2023,55(9):17-25,120. |
| YANG Yong, CHEN Haiyang, LI Yukun,et al.Progress of process development and industrial application for anhydrous hydrogen fluoride and aluminum fluoride[J].Inorganic Chemicals Industry,2023,55(9):17-25,120. | |
| [3] | 刘帅杰,姜国庆,高璐阳.磷矿伴生氟资源生产氟化氢的前景分析[J].磷肥与复肥,2023,38(6):31-36. |
| LIU Shuaijie, JIANG Guoqing, GAO Luyang.Prospect analysis of hydrogen fluoride production from fluorine resources associated with phosphate rock[J].Phosphate & Compound Fertilizer,2023,38(6):31-36. | |
| [4] | 肖晨星,高璐阳.磷矿伴生资源的利用[J].磷肥与复肥,2022,37(5):27-30,46. |
| XIAO Chenxing, GAO Luyang.Utilization of associated phosphate mineral resources[J].Phosphate & Compound Fertilizer,2022,37(5):27-30,46. | |
| [5] | 赵东,曾春华,马永强,等.对湿法磷酸脱氟渣进行循环利用的实验研究[J].硫磷设计与粉体工程,2015(4):14-17,5. |
| ZHAO Dong, ZENG Chunhua, MA Yongqiang,et al.Experimental research on recycling of fluorine removal sludge from wet-process phosphoric acid plant[J].Sulphur Phosphorus & Bulk Materials Handling Related Engineering,2015(4):14-17,5. | |
| [6] | 钟文婧,纪利俊,付全军,等.湿法磷酸脱氟渣回收技术研究[J].化学工程,2019,47(1):70-73,78. |
| ZHONG Wenjing, JI Lijun, FU Quanjun,et al.Study on recycling of the fluoride residue from wet-process phosphoric acid[J].Chemical Engineering(China),2019,47(1):70-73,78. | |
| [7] | 张程,钟文婧,纪利俊,等.湿法磷酸脱氟渣中磷和氟的浸取回收[J].化学工程,2019,47(8):1-5,21. |
| ZHANG Cheng, ZHONG Wenjing, JI Lijun,et al.Recovery of phosphorus and fluorine in defluorination sludge from wet-process phosphoric acid by leaching[J].Chemical Engineering(China),2019,47(8):1-5,21. | |
| [8] | 付子启,张程,盛勇,等.有机溶剂浸取湿法磷酸脱氟渣制备磷酸的研究[J].无机盐工业,2022,54(7):129-134. |
| FU Ziqi, ZHANG Cheng, SHENG Yong,et al.Study on preparation of phosphoric acid by leaching fluoride residue from wet-process phosphoric acid with organic solvents[J].Inorganic Chemicals Industry,2022,54(7):129-134. | |
| [9] | 盛国臣,张灿,龚孝祥.利用脱氟渣回收氟制取氟产品的方法研究[J].硫磷设计与粉体工程,2016(4):20-22,4. |
| SHENG Guochen, ZHANG Can, GONG Xiaoxiang.Study of method to prepare fluorine products with fluorine recovered from defluorination sludge[J].Sulphur Phosphorus & Bulk Materials Handling Related Engineering,2016(4):20-22,4. | |
| [10] | 王辛龙,苗林平,许德华,等.磷酸渣的资源化利用方法:CN,113277885A[P].2021-08-20. |
| [11] | DEVORE T C, GALLAHER T N.The heat of formation of Na2SiF6:A physical chemistry laboratory experiment[J].Journal of Chemical Education,1986,63(8):729. |
| [12] | 唐安江,韦德举,高珊珊,等.四氟化硅气体中杂质的检测方法[J].无机盐工业,2010,42(12):57-59. |
| TANG Anjiang, WEI Deju, GAO Shanshan,et al.Detecting methods for impurities in silicon tetrafluoride gas[J].Inorganic Chemicals Industry,2010,42(12):57-59. | |
| [13] | 张星,徐杰,王子兵,等.原料粒径对石灰石热分解反应动力学影响[J].无机盐工业,2023,55(2):79-84. |
| ZHANG Xing, XU Jie, WANG Zibing,et al.Effect of feedstock particle size on kinetics of limestone thermal decomposition reaction[J].Inorganic Chemicals Industry,2023,55(2):79-84. | |
| [14] | 周强,武斌,陈葵,等.磷尾矿热分解动力学机理与煅烧工艺研究[J].无机盐工业,2023,55(3):47-54. |
| ZHOU Qiang, WU Bin, CHEN Kui,et al.Study on thermal decomposition kinetic mechanism and calcination process of phosphorus tailings[J].Inorganic Chemicals Industry,2023,55(3):47-54. |
| [1] | XU Zijian, WANG Yu, LIU Jingyong, ZHU Chuanghai, CHEN Zhibin, HUANG Shengzheng, XIE Wuming, SUN Shuiyu. Study on defluorination process of secondary aluminum ash wet process and conversion behavior of fluoride [J]. Inorganic Chemicals Industry, 2024, 56(6): 109-118. |
| [2] | WANG Bo,ZHOU Zhixin,XU Dehua,YANG Xiushan,ZHANG Zhiye,ZHONG Yanjun,HE Binbin,JIANG Wei. Study on preparation process of feed grade DCP from sludge acid [J]. Inorganic Chemicals Industry, 2022, 54(4): 141-144. |
| [3] | Su Shu,Xu Dehua,Li Chaorong,Yang Xiushan,Wang Xinlong,Zhang Zhiye. Study on defluorination of phosphoric acid by nitric acid process [J]. Inorganic Chemicals Industry, 2021, 53(9): 24-29. |
| [4] | Bian Xiaotong,Qiu Zhaofu,Yang Ji,Yan Ruiqi,Lü Shuguang. Study on fluoride removal by modified Y zeolite particles with sodium alginate-lanthanum hydroxide [J]. Inorganic Chemicals Industry, 2020, 52(12): 86-91. |
| [5] | HE Bin-Bin, ZHOU Qiong-Bo, ZHANG Hui, FU Ying, FANG Shi-Xiang. Study on defluorination for purifying wet-process phosphoric acid by steam stripping [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(9): 49-. |
| [6] | LI Zi-Wei, ZHOU Meng, WU Ning-Lan, GONG Jia-Zhu. Globe production technology and development trend of feed-grade phosphate [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(4): 6-. |
| [7] | HE Bin-Bin, ZHANG Hui, WANG Meng-Lai, LI Xiao-Shuang, FANG Shi-Xiang, FU Ying. Study on production of industrial MAP by purification of wet-process phosphoric acid at temperature gradient [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(11): 17-. |
| [8] | CHEN Hai, ZHANG Shi-Hong, YANG Hai-Ping, LI Pan, CHEN Han-Ping, ZENG Jun. Study on thermal decomposition kinetics of limestone with large particle size [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(9): 11-. |
| [9] | YU He-Hua. Research on influencing factors of preparation of silicon tetrafluoride gas by pyrolysis from sodium fluosilicate [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(4): 46-. |
| [10] | HE Feng-Qiong. Technological transformation for production plant of sodium fluosilicate [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(3): 34-. |
| [11] | TANG Jian-Wei, DENG Huan, LIU Yong, HUA Quan-Xian. Research on resource utilization of tungsten and molybdenum beneficiation wastewater [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(8): 48-. |
| [12] | LIANG Mei-Dong, ZHOU Guo-E, YU Wen-Kai. Technical research on preparation of NaF with alkali in by-produced salt slag from hydrazine hydrate production [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(3): 48-. |
| [13] | DAI Yuan-Hua, ZENG Bo, GAO Feng, LIN Sheng-Nan-. Research on recycling of chlorine-containing wastewater by-produced from sodium fluosilicate production [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(1): 56-. |
| [14] | Zhang Mei. Research on production process of cryolite and by-product silica white by sodium hydroxide-sodium fluosilicate method [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(4): 0-0. |
| [15] | Zhang Xiaoxia;Zhang Mei;Li Jie. Method of refining industrial salt by cyclical utilization of cryolite mother solution [J]. INORGANICCHEMICALSINDUSTRY, 2009, 0(9): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||