Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (6): 101-109.doi: 10.19964/j.issn.1006-4990.2020-0411
• Reviews and Special Topics • Previous Articles Next Articles
Construction,properties and applications of two-dimensional nanofluidic channels
Liu Meili( ),Long Xiang,Tang Haiyan,Gao Banghui,Li Long,Shao Jiaojing(
),Long Xiang,Tang Haiyan,Gao Banghui,Li Long,Shao Jiaojing( )
)
- School of Materials and Metallurgy,Guizhou University,Guiyang 550025,China
-
Received:2020-07-17Online:2021-06-10Published:2021-07-08 -
Contact:Shao Jiaojing E-mail:18786125651@sina.cn;xjshao@gzu.edu.cn
CLC Number:
Cite this article
Liu Meili,Long Xiang,Tang Haiyan,Gao Banghui,Li Long,Shao Jiaojing. Construction,properties and applications of two-dimensional nanofluidic channels[J]. Inorganic Chemicals Industry, 2021, 53(6): 101-109.
share this article

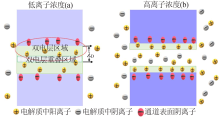
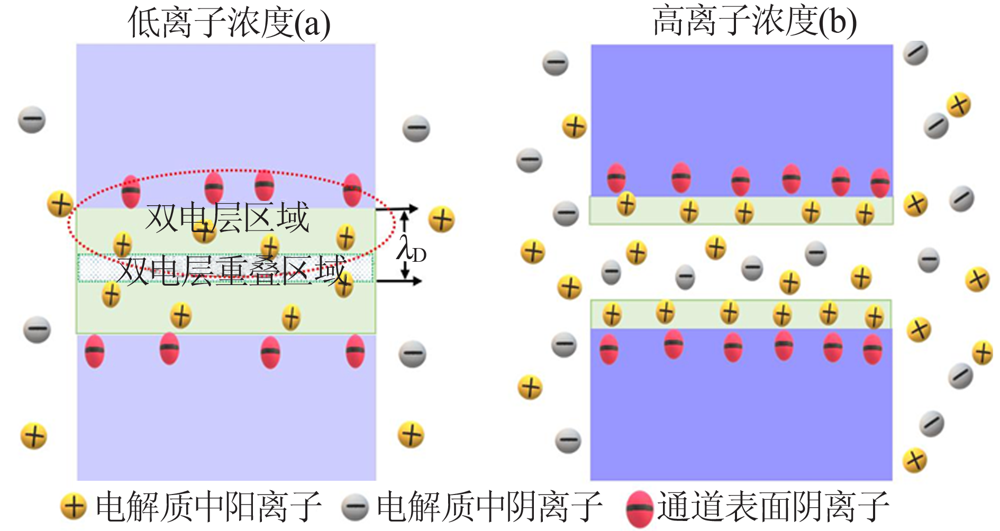
Fig.2
Schematic illustration(a) and photograph(b) of 2D nanofluidic device based on the GO/CNF membrane,and the ion conductivity as a function of the electrolyte concentration(c)[37];Proton mobilities of the GO based and the HA-modified GO based 2D nanofluidic devices(d)[38];Stability comparison of the GO(e) and CNF/GO(f) membranes in water[37];Schemes of forward(left) and reverse(right) pressure pulse inputs(g),and the as-resulted pulsed current signals(h)[40] "
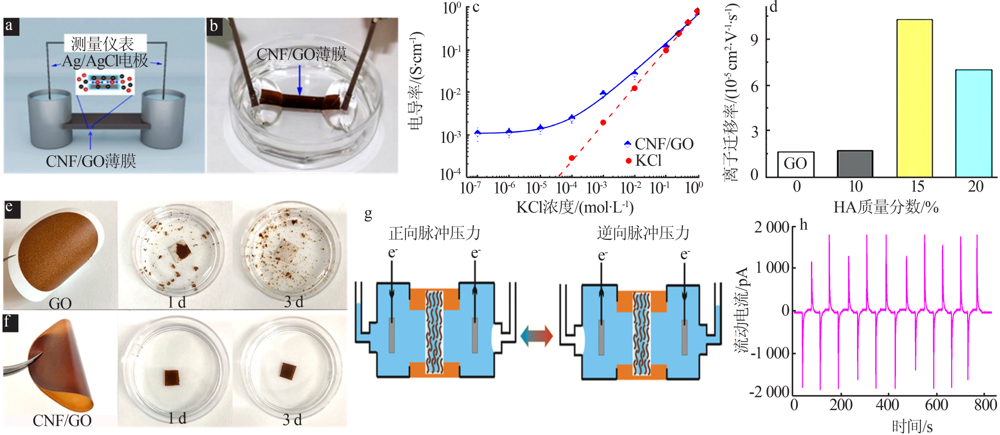
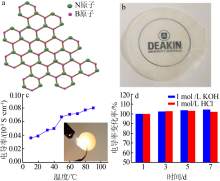
Fig.3
Structural illustration of 2D BN(a);Photo of a BN mem-brane(b)[41];Conductivity of BN membrane as the function of temperature and photo of BN membrane heated by a blaze(c);Ionic conductivity of the BN-based 2D nanochannels after immersing into 1 mol/L KOH and 1 mol/L HCl for different periods(d)[42] "
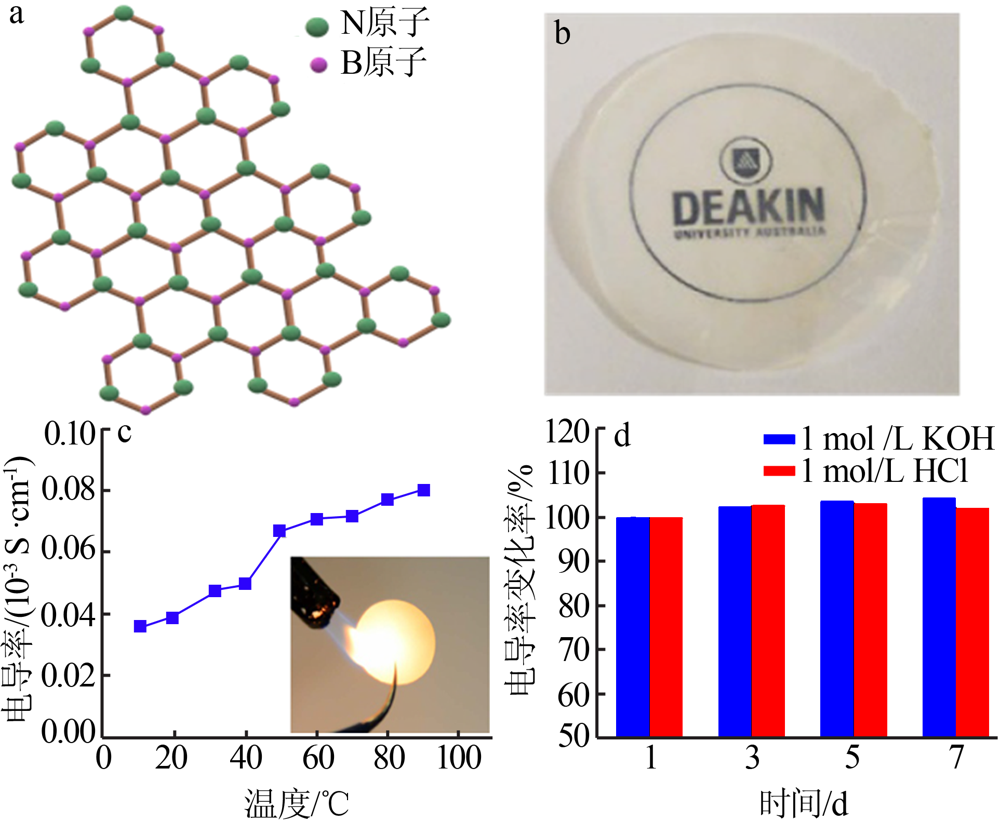
Fig.4
AFM image of the 2D vermiculite sheet and the corresponding height profile(a);Cross-sectional SEM image of a typical vermiculite membrane(b);the ion conductivity of the vermiculite-based 2D nanofluidic device as a function of the HCl electrolyte concentration(c)[43];UV-vis spectra and photo of the methylene blue solution feed and penetrate(d)[45];Schematic diagram of the working principal of the modified MMT-based nanofluidic device in response to temperature(e);voltage,and the ion currents as a function of temperature;applied voltage(f、g)[50] "
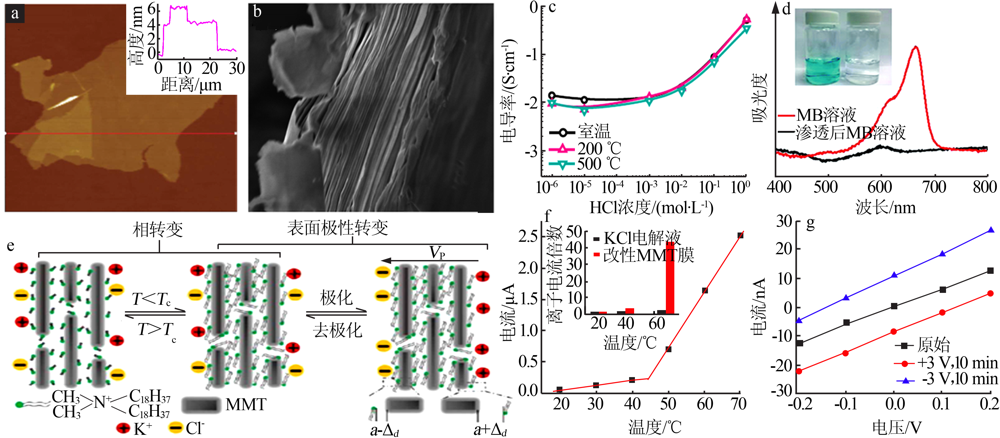

Fig.5
Schematic illustration of the preparation of Ti3C2Tx membrane(a);Permeation rate of the Ti3C2Tx and the GO membranes as the function of the cation hydrated radius(b);Water flux through the Ti3C2Tx membrane toward to water and salt solutions with different cation charge(c)[52];Structural illustration of the TMC-coated Ti2CTx-HPEI membrane(d);Sieving ability of the TMC-coated and the uncoated Ti2CTx-HPEI membranes toward to the water/isopropanol co-solvent(e)[54] "

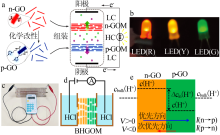
Fig.6
Schematic illustration of working principle of the RED based on n-GOM and p-GOM(a);photo of the RED-powered LEDs(b)[39];Photo of the RED assembly by ten series of co-nnected P-MXM and N-MXM powering a calculator(c)[61];scheme and(d);working principle diagram of the nano-fluidic device driven by electric field(e)[66] "
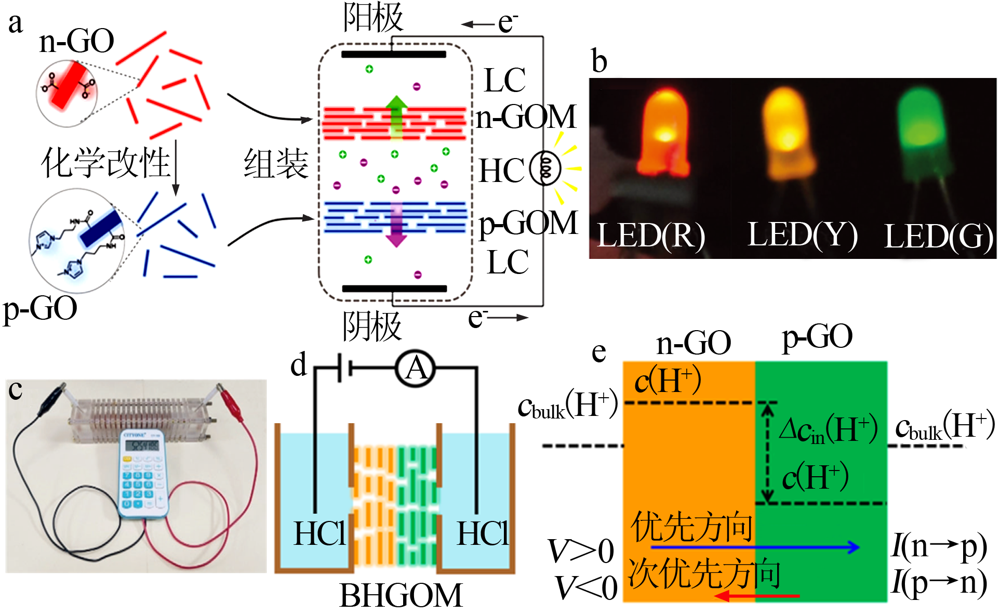
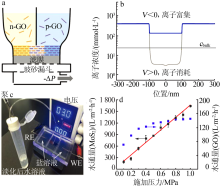
Fig.7
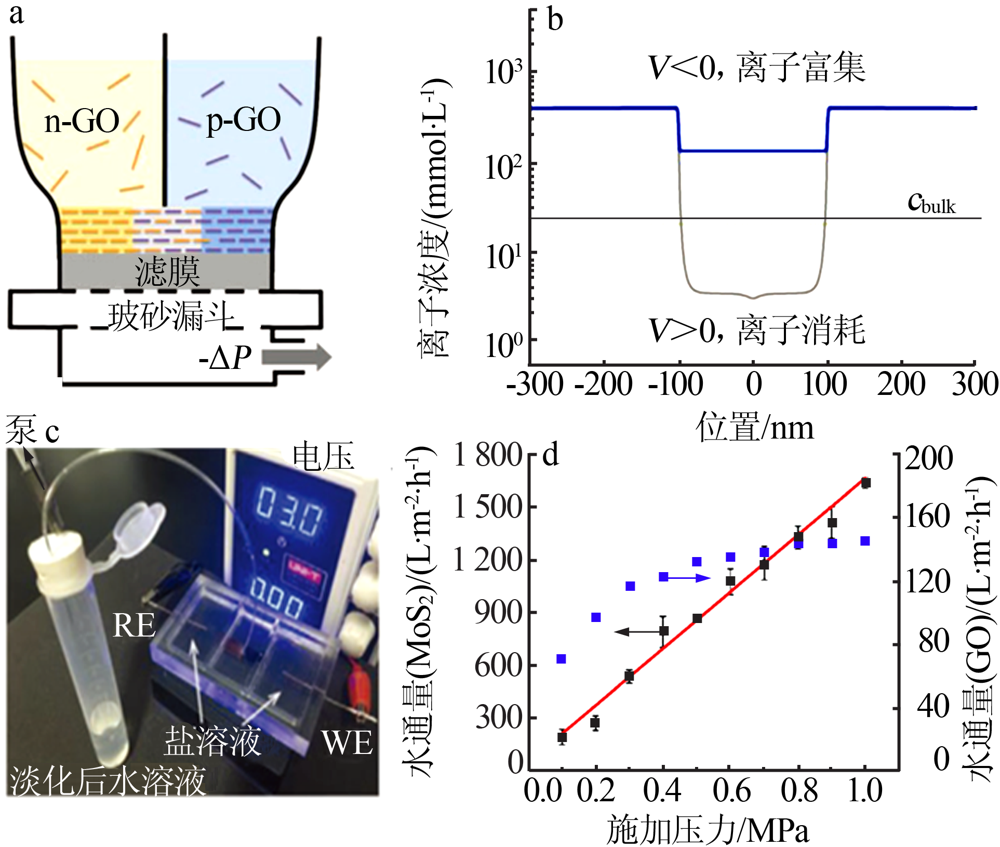
Schematic diagram of the preparation of the PHGOM(a),ion concentration of the PHGOM-based nanofluidic device as a function of the electric field direction(b),photo of the PHGOM-based desalination device(c)[75];Water flux of the MoS2 and the GO membrane based nanofluidic devices as the function of the applied pressure(d)[76] "
| [1] |
Plecis A, Schoch R B, Renaud P. Ionic transport phenomena in na-nofluidics:Experimental and theoretical study of the exclusion-en-richment effect on a chip[J]. Nano Letters, 2005,5(6):1147-1155.
doi: 10.1021/nl050265h |
| [2] |
Karnik R, Fan R, Yue M, et al. Electrostatic control of ions and mo-lecules in nanofluidic transistors[J]. Nano Letters, 2005,5(5):943-948.
doi: 10.1021/nl050493b |
| [3] | Stein D, Kruithof M. Surface-charge-governed ion transport in nano-fluidic channels[J]. Physical Review Letters, 2004,93(3).Doi: 10.1103/PhysRevLett.93.035901. |
| [4] |
Han Y, Xu Z, Gao C. Ultrathin graphene nanofiltration membrane for water purification[J]. Advanced Functional Materials, 2013,23(29):3693-3700.
doi: 10.1002/adfm.v23.29 |
| [5] |
Huang H, Mao Y, Ying Y, et al. Salt concentration,pH and pressure controlled separation of small molecules through lamellar graphene oxide membranes[J]. Chemical Communications, 2013,49(53):5963-5965.
doi: 10.1039/c3cc41953c |
| [6] | Wang S, Yang L, He G, et al. Two-dimensional nanochannel mem-branes for molecular and ionic separations[J]. Chemical Society Re-views, 2020,49(4):1071-1089. |
| [7] | 郭维, 江雷. 基于仿生智能纳米孔道的先进能源转换体系[J]. 中国科学:化学, 2011,41(8):1257-1270. |
| [8] | Osterle J F. A unified treatment of the thermodynamics of steady-st-ate energy conversion[J]. Flow Turbulence and Combustion, 1964,12(6):425-434. |
| [9] | Duan C H, Wang W, Xie Q. Review article:fabrication of nanofluidic devices[J]. Biomicrofluidics, 2013,7(2).Doi: 10.1063/1.4794973. |
| [10] |
Li W, Tegenfeldt J O, Chen L, et al. Sacrificial polymers for nanofl-uidic channels in biological applications[J]. Nanotechnology, 2003,14(6):578-583.
doi: 10.1088/0957-4484/14/6/302 |
| [11] | Yang R, Lu B, Wan J, et al. Fabrication of micro/nano fluidic chan-nels by nanoimprint lithography and bonding using SU-8[J]. Mi-croelectronic Engineering, 2009,86(4):1379-1381. |
| [12] | Raidongia K, Huang J. Nanofluidic ion transport through reconst-ructed layered materials[J]. Journal of the American Chemical So-ciety, 2012,134(40):16528-16531. |
| [13] |
Zhang H. Ultrathin two-dimensional nanomaterials[J]. ACS Nano, 2015,9(10):9451-9469.
doi: 10.1021/acsnano.5b05040 pmid: 26407037 |
| [14] | Novoselov K S, Jiang D, Schedin F, et al. Two-dimensional atomic crystals[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005,102(30):10451-10453. |
| [15] |
Li H, Wu J, Yin Z, et al. Preparation and applications of mechani-cally exfoliated single-layer and multilayer MoS2 and WSe2 nano-sheets[J]. Accounts of Chemical Research, 2014,47(4):1067-1075.
doi: 10.1021/ar4002312 |
| [16] | Castellanosgomez A, Vicarelli L, Prada E, et al. Isolation and char-acterization of few-layer black phosphorus[J]. 2D Materials, 2014,1(2).Doi: 10.1088/2053-1583/1/2/025001. |
| [17] | Hernandez Y, Nicolosi V, Lotya M, et al. High-yield production of graphene by liquid-phase exfoliation of graphite[J]. Nature Nano-technology, 2008,3(9):563-568. |
| [18] |
Khan U, May P, O′neill A, et al. Polymer reinforcement using liq-uid-exfoliated boron nitride nanosheets[J]. Nanoscale, 2013,5(2):581-587.
doi: 10.1039/C2NR33049K |
| [19] |
Brent J R, Savjani N, Lewis E A, et al. Production of few-layer pho-sphorene by liquid exfoliation of black phosphorus[J]. Chemical Communications, 2014,50(87):13338-13341.
doi: 10.1039/C4CC05752J |
| [20] | Yu J X, Li J, Zhang W F, et al. Synjournal of high quality two-dimen-sional materials via chemical vapor deposition[J]. Chemical Sci-ence, 2015,6(12):6705-6716. |
| [21] |
Zhang L, Gu F, Tong L, et al. Simple and cost-effective fabrication of two-dimensional plastic nanochannels from silica nanowire templates[J]. Microfluidics and Nanofluidics, 2008,5(6):727-732.
doi: 10.1007/s10404-008-0314-4 |
| [22] | Shao J J, Zheng D Y, Li Z J, et al. Top-down fabrication of two-di-mensional nanomaterials:Controllable liquid phase exfoliation[J]. New Carbon Materials, 2016,31(2):97-114. |
| [23] |
Koltonow A R, Huang J X. Ionic transport two-dimensional nanofl-uidics[J]. Science, 2016,351(6280):1395-1396.
doi: 10.1126/science.aaf5289 |
| [24] |
Novoselov K S, Geim A K, Morozov S V, et al. Electric field effect in atomically thin carbon films[J]. Science, 2004,306(5696):666-669.
doi: 10.1126/science.1102896 |
| [25] |
Gao W, Alemany L B, Ci L, et al. New insights into the structure and reduction of graphite oxide[J]. Nature Chemistry, 2009,1(5):403-408.
doi: 10.1038/nchem.281 |
| [26] |
Szabó T, Berkesi O, Forgó P, et al. Evolution of surface functional groups in a series of progressively oxidized graphite oxides[J]. Chemistry of Materials, 2006,18(11):2740-2749.
doi: 10.1021/cm060258+ |
| [27] | Guo W, Tian Y, Jiang L. Asymmetric ion transport through ion-chan-nel-mimetic solid-state nanopores[J]. Accounts of Chemical Re-search, 2013,46(12):2834-2846. |
| [28] |
Siwy Z S. Ion-current rectification in nanopores and nanotubes with broken symmetry[J]. Advanced Functional Materials, 2006,16(6):735-746.
doi: 10.1002/(ISSN)1616-3028 |
| [29] | Miansari M, Friend J R, Yeo L Y. Enhanced ion current rectifica-tion in 2D graphene-based nanofluidic devices[J]. Advanced Sci-ence, 2015,2(6).Doi: 10.1002/advs.201500062. |
| [30] | Wang C, Liu F, Tan Z, et al. Fabrication of bio-inspired 2D MOFs/ PAA hybrid membrane for asymmetric ion transport[J]. Advanced Functional Materials, 2020,30(9).Doi: 10.1002/adfm.201908804. |
| [31] |
Zhang H, Hou X, Yang Z, et al. Bio-inspired smart single asymme-tric hourglass nanochannels for continuous shape and ion transpo-rt control[J]. Small, 2015,11(7):786-791.
doi: 10.1002/smll.201401677 |
| [32] |
Zhang Z, Li P, Kong X Y, et al. Bioinspired heterogeneous ion pump membranes:Unidirectional selective pumping and controllable gat-ing properties stemming from asymmetric ionic group distribu-tion[J]. Journal of the American Chemical Society, 2018,140(3):1083-1090.
doi: 10.1021/jacs.7b11472 |
| [33] |
Qiao Y, Lu J, Ma W, et al. Optoelectronic modulation of ionic con-ductance and rectification through a heterogeneous 1D/2D nano-fluidic membrane[J]. Chemical Communications, 2020,56(24):3508-3511.
doi: 10.1039/D0CC00082E |
| [34] | Zhang X, Jia M, Wang L, et al. Rectified Ion transport through 2D nanofluidic heterojunctions[J]. Physica Status Solidi-Rapid Rese-arch Letters, 2019,13(7).Doi: 10.1002/pssr.201900129. |
| [35] |
Gao J, Koltonow A R, Raidongia K, et al. Kirigami nanofluidics[J]. Materials Chemistry Frontiers, 2018,2(3):475-482.
doi: 10.1039/C7QM00620A |
| [36] |
Wang L, Feng Y, Zhou Y, et al. Photo-switchable two-dimensional nanofluidic ionic diodes[J]. Chemical Science, 2017,8(6):4381-4386.
doi: 10.1039/C7SC00153C |
| [37] |
Wang C, Miao C, Zhu X, et al. Fabrication of stable and flexible nanocomposite membranes comprised of cellulose nanofibers and graphene oxide for nanofluidic ion transport[J]. ACS Applied Nano Materials, 2019,2(7):4193-4202.
doi: 10.1021/acsanm.9b00652 |
| [38] |
Konch T J, Gogoi R K, Gogoi A, et al. Nanofluidic transport through humic acid modified graphene oxide nanochannels[J]. Materials Chemistry Frontiers, 2018,2(9):1647-1654.
doi: 10.1039/C8QM00272J |
| [39] | Ji J, Kang Q, Zhou Y, et al. Osmotic power generation with positively and negatively charged 2D nanofluidic membrane pairs[J]. Advan-ced Functional Materials, 2017,27(2).Doi: 10.1002/adfm.201603623. |
| [40] |
Guo W, Cheng C, Wu Y, et al. Bio-inspired two-dimensional nano-fluidic generators based on a layered graphene hydrogel membr-ane[J]. Advanced Materials, 2013,25(42):6064-6068.
doi: 10.1002/adma.201302441 |
| [41] | Lei W, Mochalin V N, Liu D, et al. Boron nitride colloidal solutions, ultralight aerogels and freestanding membranes through one-step exfoliation and functionalization[J]. Nature Communications, 2015,6.Doi: 10.1038/ncomms9849. |
| [42] | Qin S, Liu D, Wang G, et al. High and stable ionic conductivity in 2D nanofluidic ion channels between boron nitride layers[J]. Jo-urnal of the American Chemical Society, 2017,139(18):6314-6320. |
| [43] | Shao J J, Raidongia K, Koltonow A R, et al. Self-assembled two-di-mensional nanofluidic proton channels with high thermal stabili-ty[J]. Nature Communications, 2015,6.Doi: 10.1038/ncomms8602. |
| [44] | Liu M L, Huang M, Tian L Y, et al. Two-dimensional nanochannel arrays based on flexible montmorillonite membranes[J]. ACS App-plied Materials & Interfaces, 2018,10(51):44915-44923. |
| [45] |
Zhou Y, Ding H, Smith A T, et al. Nanofluidic energy conversion and molecular separation through highly stable clay-based mem-branes[J]. Journal of Materials Chemistry A, 2019,7(23):14089-14096.
doi: 10.1039/c9ta00801b |
| [46] |
Zhang M, Hou X, Wang J, et al. Light and pH cooperative nanoflui-dic diode using a spiropyran-functionalized single nanochannel[J]. Advanced Materials, 2012,24(18):2424-2428.
doi: 10.1002/adma.201104536 |
| [47] |
Zhang Z, Xie G, Xiao K, et al. Asymmetric multifunctional hetero-geneous membranes for ph-and temperature-cooperative smart ion transport modulation[J]. Advanced Materials, 2016,28(43):9613-9619.
doi: 10.1002/adma.201602758 |
| [48] |
Newton M R, Bohaty A K, Zhang Y, et al. pH- and ionic strength-controlled cation permselectivity in amine-modified nanoporous opal films[J]. Langmuir, 2006,22(9):4429-4432.
doi: 10.1021/la053069z |
| [49] |
Zhang Q, Liu Z, Wang K, et al. Organic/inorganic hybrid nanochan-nels based on polypyrrole-embedded alumina nanopore arrays:pH- and light-modulated ion transport[J]. Advanced Functional Materials, 2015,25(14):2091-2098.
doi: 10.1002/adfm.v25.14 |
| [50] |
Xiao T, Liu Q, Zhang Q, et al. Temperature and voltage dual-respo-nsive ion transport in bilayer-intercalated layered membranes with 2D nanofluidic channels[J]. Journal of Physical Chemistry C, 2017,121(34):18954-18961.
doi: 10.1021/acs.jpcc.7b06245 |
| [51] |
Naguib M, Mochalin V, Barsoum M W, et al. MXenes:A new family of two-dimensional materials[J]. Advanced Materials, 2014,26(7):992-1005.
doi: 10.1002/adma.201304138 |
| [52] |
Ren C E, Hatzell K B, Alhabeb M, et al. Charge- and size-selective ion sieving through Ti3C2Tx MXene membranes[J]. The Journal of Physical Chemistry Letters, 2015,6(20):4026-4031.
doi: 10.1021/acs.jpclett.5b01895 |
| [53] |
Hope M A, Forse A C, Griffith K J, et al. NMR reveals the surface functionalisation of Ti3C2 MXene[J]. Physical Chemistry Chemical Physics, 2016,18(7):5099-5102.
doi: 10.1039/C6CP00330C |
| [54] |
Liu G, Shen J, Ji Y, et al. Two-dimensional Ti2CTx MXene membr-anes with integrated and ordered nanochannels for efficient solvent dehydration[J]. Journal of Materials Chemistry A, 2019,7(19):12095-12104.
doi: 10.1039/C9TA01507H |
| [55] |
Huang S, Mochalin V. Hydrolysis of 2D transition-metal carbides (MXenes)in colloidal solutions[J]. Inorganic Chemistry, 2019,58(3):1958-1966.
doi: 10.1021/acs.inorgchem.8b02890 |
| [56] |
Zhao X, Vashisth A, Prehn E, et al. Antioxidants unlock shelf-stable Ti3C2Tx(MXene) nanosheet dispersions[J]. Matter, 2019,1(2):513-526.
doi: 10.1016/j.matt.2019.05.020 |
| [57] | Wu C, Unnikrishnan B, Chen I P, et al. Excellent oxidation resistive MXene aqueous ink for micro-supercapacitor application[J]. En-ergy Storage Materials, 2020,25:563-571. |
| [58] | Natu V, Hart J L, Sokol M, et al. Edge capping of 2D-MXene sheets with polyanionic salts to mitigate oxidation in aqueous colloidal su-spensions[J]. Angewandte Chemie, 2019,131(36):12655-12660. |
| [59] | Cheng H, Zhou Y, Feng Y, et al. Electrokinetic energy conversion in self-assembled 2D nanofluidic channels with janus nanobuild-ing blocks[J]. Advanced Materials, 2017,29(23).Doi: 10.1002/adma.201700177. |
| [60] |
Hong S, Ming F, Shi Y, et al. Two-dimensional Ti3C2Tx MXene me-mbranes as nanofluidic osmotic power generators[J]. ACS Nano, 2019,13(8):8917-8925.
doi: 10.1021/acsnano.9b02579 |
| [61] | Ding L, Xiao D, Lu Z, et al. Oppositely charged Ti3C2Tx MXene mem-branes with 2D nanofluidic channels for osmotic energy harvest-ing[J]. Angewandte Chemie, 2020,59(22):8720-8726. |
| [62] | Cao L, Xiao F, Feng Y, et al. Anomalous channel-length depende-nce in nanofluidic osmotic energy conversion[J]. Advanced Func-tional Materials, 2017,27(9).Doi: 10.1002/adfm.201604302. |
| [63] | Siwy Z, Kosińska I, Fuliński A, et al. Asymmetric diffusion through synthetic nanopores[J]. Physical Review Letters, 2005,94(4).Doi: 10.1103/PhysRevLett.94.048102. |
| [64] |
Dlugolecki P, Nymeijer K, Metz S J, et al. Current status of ion ex-change membranes for power generation from salinity gradients[J]. Journal of Membrane Science, 2008,319(1):214-222.
doi: 10.1016/j.memsci.2008.03.037 |
| [65] |
Wen L, Tian Y, Guo Y, et al. Conversion of light to electricity by photoinduced reversible pH changes and biomimetic nanofluidic channels[J]. Advanced Functional Materials, 2013,23(22):2887-2893.
doi: 10.1002/adfm.201203259 |
| [66] |
Zhang X, Wen Q, Wang L, et al. Asymmetric electrokinetic proton transport through 2D nanofluidic heterojunctions[J]. ACS Nano, 2019,13(4):4238-4245.
doi: 10.1021/acsnano.8b09285 |
| [67] | Wang L, Wen Q, Jia P, et al. Light-driven active proton transport through photoacid- and photobase-doped janus graphene oxide membranes[J]. Advanced Materials, 2019,31(36).Doi: 10.1002/adma.201903029. |
| [68] |
White W, Sanborn C D, Reiter R S, et al. Observation of photovolt-aic action from photoacid-modified nafion due to light-driven ion transport[J]. Journal of the American Chemical Society, 2017,139(34):11726-11733.
doi: 10.1021/jacs.7b00974 |
| [69] |
Yang X W, Cheng C, Wang Y F, et al. Liquid-mediated dense inte-gration of graphene materials for compact capacitive energy storage[J]. Science, 2013,341(6145):534-537.
doi: 10.1126/science.1239089 |
| [70] |
Joshi R, Carbone P, Wang F C, et al. Precise and ultrafast molec-ular sieving through graphene oxide membranes[J]. Science, 2014,343(6172):752-754.
doi: 10.1126/science.1245711 |
| [71] | Su Y, Kravets V G, Wong S L, et al. Impermeable barrier films and protective coatings based on reduced graphene oxide[J]. Nature Communications, 2014,5(1).Doi: 10.1038/ncomms5843. |
| [72] | Lin L, Grossman J C. Atomistic understandings of reduced graphene oxide as an ultrathin-film nanoporous membrane for separations[J]. Nature Communications, 2015,6(1).Doi: 10.1038/ncomms9335. |
| [73] |
Chen L, Shi G, Shen J, et al. Ion sieving in graphene oxide membr-anes via cationic control of interlayer spacing[J]. Nature, 2017,550(7676):380-383.
doi: 10.1038/nature24044 |
| [74] |
Sun P, Zheng F, Zhu M, et al. Selective trans-membrane transport of alkali and alkaline earth cations through graphene oxide mem-branes based on cation-π interactions[J]. ACS Nano, 2014,8(1):850-859.
doi: 10.1021/nn4055682 |
| [75] | Wen Q, Jia P, Cao L, et al. Electric-field-induced ionic sieving at planar graphene oxide heterojunctions for miniaturized water desa-lination[J]. Advanced Materials, 2020,32(16).Doi: 10.1002/adma.201903954. |
| [76] |
Sun L, Huang H, Peng X. Laminar MoS2 membranes for molecule separation[J]. Chemical Communications, 2013,49(91):10718-10720.
doi: 10.1039/c3cc46136j |
| [77] |
Sun L, Ying Y, Huang H, et al. Ultrafast molecule separation thro-ugh layered WS2 nanosheet membranes[J]. ACS Nano, 2014,8(6):6304-6311.
doi: 10.1021/nn501786m |
| [78] | Yang J, Hu X, Kong X, et al. Photo-induced ultrafast active ion tr-ansport through graphene oxide membranes[J]. Nature Communications, 2019,10(1).Doi: 10.1038/s41467-019-09178-x. |
| [1] | Du Miao,Zhang Xin. Progress in application research of two-dimensional nanomaterials in water treatment [J]. Inorganic Chemicals Industry, 2020, 52(1): 17-21. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||