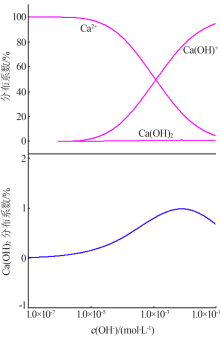
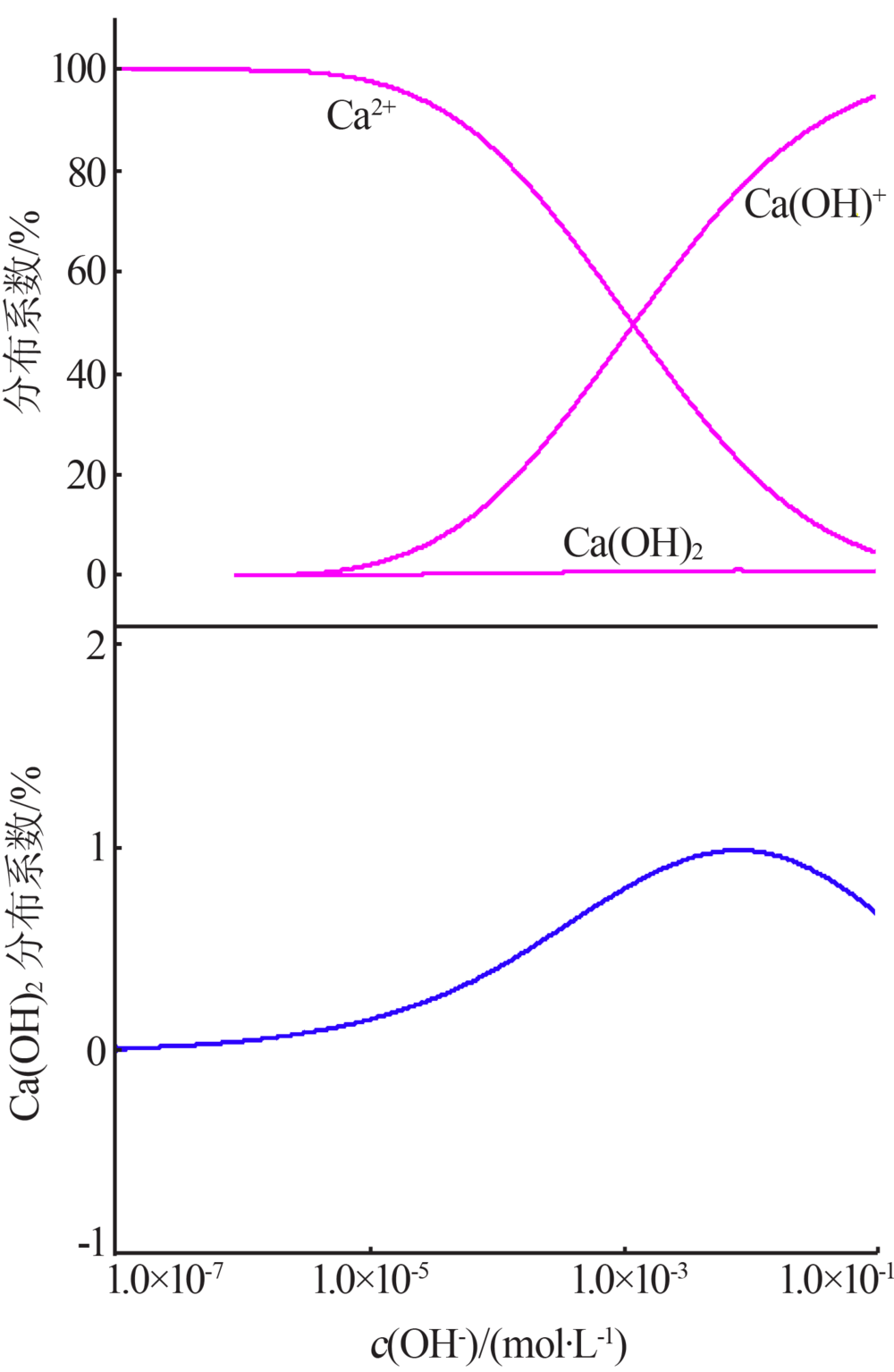
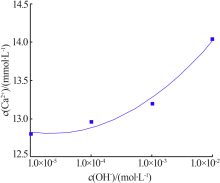
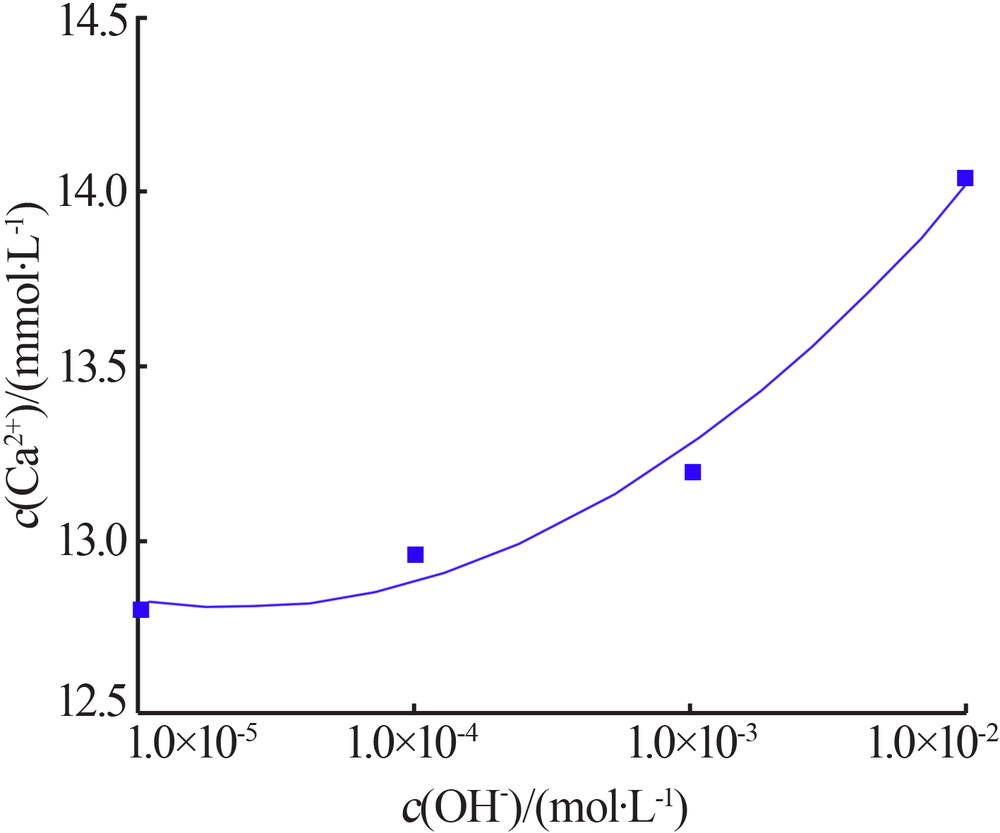
| [1] |
张凡凡, 陈超, 张西兴, 等. 不同来源石膏的性能特点与应用分析[J]. 无机盐工业, 2017,49(8):10-13.
|
| [2] |
Mao X L, Song X F, Lu G M, et al. Effects of metal ions on crystal morphology and size of calcium sulfate whiskers in aqueous HCl solutions[J]. Industrial & Engineering Chemistry Research, 2014,53(45):17625-17635.
|
| [3] |
Liu T J, Fan H, Xu Y X, et al. Effects of metal ions on the morpho-logy of calcium sulfate hemihydrate whiskers by hydrothermal met-hod[J]. Frontiers of Chemical Science and Engineering, 2017,11(4):545-553.
doi: 10.1007/s11705-017-1665-8
|
| [4] |
Luo K B, Li H P, Tan Y X.Study on the preparation of calcium sul-fate whisker by hydrothermal method[J].Advanced Materials Rese-arch, 2013,602/603/604:1369-1372.
|
| [5] |
王宇斌, 文堪, 王森, 等. 同离子效应对半水硫酸钙形貌的调控机理[J]. 高校化学工程学报, 2018,32(6):1444-1449.
|
| [6] |
王鑫, 纪利俊, 陈葵, 等. pH与Ca2+浓度对磷石膏常压制备α-半水硫酸钙的影响[J]. 无机盐工业, 2017,49(11):54-58.
|
| [7] |
史培阳, 邓志银, 袁义义, 等. 利用脱硫石膏水热合成硫酸钙晶须[J]. 东北大学学报:自然科学版, 2010,31(1):76-79.
|
| [8] |
邓志银, 袁义义, 孙骏, 等. pH值对脱硫石膏晶须生长行为的影响[J]. 过程工程学报, 2009,9(6):1142-1146.
|
| [9] |
Guan B H, Shen Z X, Wu Z B, et al. Effect of pH on the preparation of α-calcium sulfate hemihydrate from FGD gypsum with the hydro-drothermal method[J]. Journal of the American Ceramic Society, 2008,91(12):3835-3840.
doi: 10.1111/jace.2008.91.issue-12
|
| [10] |
王微, 刘代俊, 陈建钧. 杂质对石膏晶须制备的影响[J]. 无机盐工业, 2016,48(4):31-34.
|
| [11] |
天津大学物理化学教研室. 物理化学(简明版)[M]. 北京: 高等教育出版社, 2010: 234-240.
|
| [12] |
袁致涛, 王晓丽, 韩跃新, 等. 水热法合成超细硫酸钙晶须[J]. 东北大学学报:自然科学版, 2008,29(4):573-576.
|
 ),Zhang Lu(
),Zhang Lu( ),Mao Xinyu,Wang Wangbo,Dang Weiben
),Mao Xinyu,Wang Wangbo,Dang Weiben