Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (11): 1-9.doi: 10.19964/j.issn.1006-4990.2020-0674
• Reviews and Special Topics • Next Articles
Research progress on pH-universal MoS2-based materials for electrocatalytic hydrogen evolution
GONG Feilong( ),LIU Yuheng,LIU Mengmeng,WANG Guoqing(
),LIU Yuheng,LIU Mengmeng,WANG Guoqing( )
)
- Key Laboratory of Surface and Interface Science and Technology,College of Materials and Chemical Engineering,Zhengzhou University of Light Industry,Zhengzhou 450002,China
-
Received:2020-12-14Online:2021-11-10Published:2021-11-15 -
Contact:WANG Guoqing E-mail:gfl@zzuli.edu.cn;1989008@zzuli.edu.cn;2018806@zzuli.edu.cn
CLC Number:
Cite this article
GONG Feilong,LIU Yuheng,LIU Mengmeng,WANG Guoqing. Research progress on pH-universal MoS2-based materials for electrocatalytic hydrogen evolution[J]. Inorganic Chemicals Industry, 2021, 53(11): 1-9.
share this article
| [1] | YU J C. Research on China′s new energy development path based on circular economy[J]. Academic Journal of Business & Management, 2020, 2(3):49-53. |
| [2] | 赵扬, 彭鸣, 谭勇文. 三维自支撑电极在析氢反应中的研究进展[J]. 中国材料进展, 2018, 37(4):241-253,281. |
| [3] | 邱雯曦, 钟辉云, 周礼仕, 等. 基于过渡金属电催化析氢催化剂的研究进展[J]. 工业催化, 2020, 28(9):8-16. |
| [4] | DINCER I, ACAR C. Review and evaluation of hydrogen production methods for better sustainability[J]. International Journal of Hydro-gen Energy, 2015, 40(34):11094-11111. |
| [5] | 王培灿, 雷青, 刘帅, 等. 电解水制氢MoS2催化剂研究与氢能技术展望[J]. 化工进展, 2019, 38(1):278-290. |
| [6] | 李凯迪, 王思佳, 朱鑫瑞, 等. MoS2/核桃壳活性炭复合纳米材料的制备及其电催化析氢性能研究[J]. 化工新型材料, 2019, 47(4):71-75. |
| [7] | 胡颖. 二硫化钼用于电催化析氢反应的研究进展[J]. 新材料产业, 2017(10):43-49. |
| [8] |
LIU Q, WANG E, SUN G. Layered transition-metal hydroxides for alkaline hydrogen evolution reaction[J]. Chinese Journal of Catalysis, 2020, 41(4):574-591.
doi: 10.1016/S1872-2067(19)63458-3 |
| [9] |
ZHAO G, RUI K, DOU S X, et al. Heterostructures for electrochemi-cal hydrogen evolution reaction:A review[J]. Advanced Functional Materials, 2018, 28(43).Doi: 10.1002/adfm.201803291.
doi: 10.1002/adfm.201803291 |
| [10] |
VESBORG P; BRIAN S; CHORKENDORFF I. Recent development in hydrogen evolution reaction catalysts and their practical imple-mentation[J]. The Journal of Physical Chemistry Letters, 2015, 6(6):951-957.
doi: 10.1021/acs.jpclett.5b00306 |
| [11] |
SINHA A, DHANJAI, JAIN R, et al. Voltammetric sensing based on the use of advanced carbonaceous nanomaterials:A review[J]. Microchimica Acta, 2018, 185(2).Doi: 10.1007/s0604-017-2626-0.
doi: 10.1007/s0604-017-2626-0 |
| [12] | 马浩, 杨瑞霞, 李春静. 层状二硫化钼材料的制备和应用进展[J]. 材料导报, 2017(3):7-14. |
| [13] | 宋晓琳, 陈贵锋, 关丽秀, 等. MoS2为基纳米复合材料的制备及性能研究进展[J]. 中国材料进展, 2017, 36(12):929-937,949. |
| [14] | 吴瑞峰, 王宏伟, 皮晓媛. 二硫化钼复合导电碳作为高性能钠离子电池负极材料的研究[J]. 无机盐工业, 2019, 51(7):28-32. |
| [15] | 杜淼, 张馨. 二维纳米材料在水处理中的应用研究进展[J]. 无机盐工业, 2020, 52(1):17-21. |
| [16] | 王毅, 李刚, 李朋伟, 等. 层状二硫化钼光催化产氢的研究进展[J]. 半导体光电, 2016, 37(4):461-466. |
| [17] |
TSAI C, ABILD-PEDERSEN F, NORSKOV J K. Tuning the MoS2 edge-site activity for hydrogen evolution via support interactio-ns[J]. Nano Letters, 2014, 14(3):1381-1387.
doi: 10.1021/nl404444k |
| [18] | 郭亚肖, 商昌帅, 李敬, 等. 电催化析氢、析氧及氧还原的研究进展[J]. 中国科学:化学, 2018, 48(8):926-940. |
| [19] | YIN J, FAN Q, LI Y, et al. Ni-C-N nanosheets as catalyst for hy-drogen evolution reaction[J]. Journal of the American Chemical So-ciety, 2016, 138(44):14546-14549. |
| [20] |
WANG S, WANG J, ZHU M, et al. Molybdenum-carbide-modified nitrogen-doped carbon vesicle encapsulating nickel nanoparticles:A highly efficient,low-cost catalyst for hydrogen evolution reac-tion[J]. Journal of the American Chemical Society, 2015, 137(50):15753-15759.
doi: 10.1021/jacs.5b07924 |
| [21] |
GE X B, CHEN L Y, ZHANG L, et al. Nanoporous metal enhanced catalytic activities of amorphous molybdenum sulfide for high-effi-ciency hydrogen production[J]. Advanced Materials, 2014, 26(19):3100-3104.
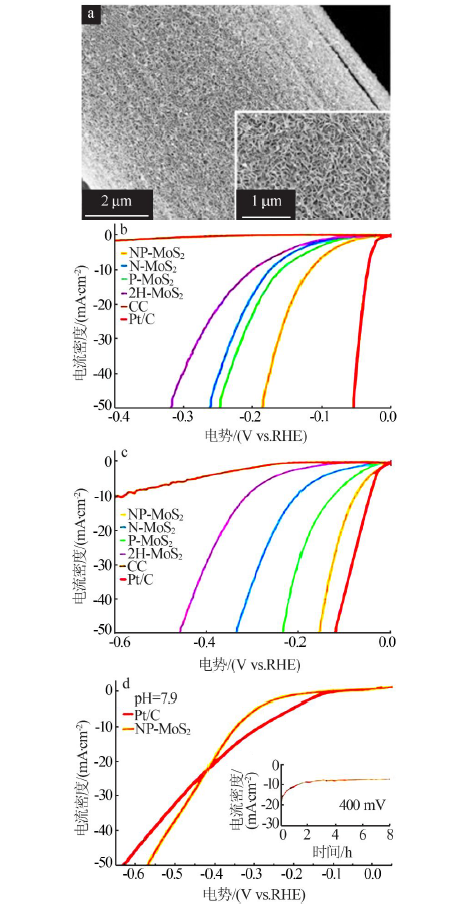
doi: 10.1002/adma.201305678 |
| [22] | 孙娇娇, 郭文锋, 唐永福, 等. 纳米花状MoS2的水热法合成及其电催化析氢性能[J]. 中国科技论文, 2017, 12(12):1395-1398. |
| [23] | 张浩翔, 胡芳仁, 郭俊宏. 水热法合成纳米二硫化钼及其电催化性析氢性能研究[J], 功能材料, 2019, 50(11):11128-11132. |
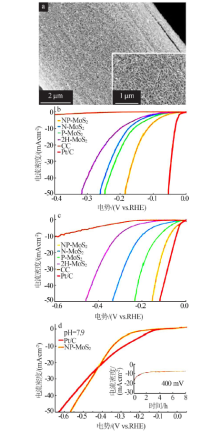
| [24] | 张洪格, 朱佳, 章永凡. Zn掺杂MoS2的构型、电子结构及电催化析氢性能的理论研究[J]. 江西师范大学学报:自然科学版, 2020, 44(4):417-423. |
| [25] |
SUN Y, LI C, ZHANG J, et al. First-principles study of the catalytic properties of Co-doped molybdenum disulfide nanoribbons for the hydrogen evolution reaction[J]. Journal of Applied Physics, 2020, 128(4).Doi: 10.1063/5.0007906.
doi: 10.1063/5.0007906 |
| [26] | 侯世成, 任王瑜, 朱清, 等. Ni掺杂MoS2/石墨烯催化剂的制备及其电催化析氢活性[J]. 浙江大学学报:工学版, 2019, 53(8):1610-1617. |
| [27] |
BOLAR S, SHIT S, MURMU N C, et al. FeNiSx@MoS2 heterostruc-ture:A bioinspired nonprecious electrocatalyst for the hydrogen evolution reaction in acidic and basic media[J]. Chemelectrochem, 2020, 7(15):3324-3335.
doi: 10.1002/celc.v7.15 |
| [28] |
张宇, 王世兴, 杨蕊, 等. Co9S8/MoS2异质结构的构筑及电催化析氢性能研究[J]. 化学学报, 2020, 78(12):1455-1460.
doi: 10.6023/A20070332 |
| [29] |
LIN J, WANG P, WANG H, et al. Defect-rich heterogeneous MoS2/NiS2 nanosheets electrocatalysts for efficient overall water splitt-ing[J]. Advanced Science, 2019, 6(14).Doi: 10.1002/advs.201900246.
doi: 10.1002/advs.201900246 |
| [30] |
LUKOWSKI M A, DANIEL A S, MENG F, et al. Enhanced hydro-gen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets[J]. Journal of the American Chemical Society, 2013, 135(28):10274-10277.
doi: 10.1021/ja404523s |
| [31] |
ZHOU Q, LUO X, LI Y, et al. A feasible and environmentally frien-endly method to simultaneously synthesize MoS2 quantum dots and pore-rich monolayer MoS2 for hydrogen evolution reaction[J]. International Journal of Hydrogen Energy, 2020, 45(1):433-442.
doi: 10.1016/j.ijhydene.2019.10.167 |
| [32] | XU S, LI D, WU P. One-pot,facile,and versatile synjournal of mono-layer MoS2/WS2 quantum dots as bioimaging probes and efficient electrocatalysts for hydrogen evolution reaction[J]. Advanced Fu-nctional Materials, 2015, 25(7):1127-1136. |
| [33] |
WU Z, FANG B, WANG Z, et al. MoS2 nanosheets:A designed st-structure with high active site density for the hydrogen evolution Reaction[J]. ACS Catalysis, 2013, 3(9):2101-2107.
doi: 10.1021/cs400384h |
| [34] |
ZHANG J, WU J, GUO H, et al. Unveiling active sites for the hydro-gen evolution reaction on monolayer MoS2[J]. Advanced Materials, 2017, 29(42).Doi: 10.1002/adma.201701955.
doi: 10.1002/adma.201701955 |
| [35] |
LI H, TSAI C, KOH A L, et al. Activating and optimizing MoS2 ba-sal planes for hydrogen evolution through the formation of strained sulphur vacancies[J]. Nature Materials, 2016, 15(1):48-53.
doi: 10.1038/nmat4465 |
| [36] |
CHEN Y, RONG J, TAO Q, et al. Modifying microscopic structures of MoS2 by high pressure and high temperature used in hydrogen evolution reaction[J]. Electrochimica Acta, 2020, 357.Doi: 10.1016/j.electacta.2020.136868.
doi: 10.1016/j.electacta.2020.136868 |
| [37] |
ANJUM M A R, JEONG H Y, LEE M H, et al. Efficient hydrogen evolution reaction catalysis in alkaline media by all-in-one MoS2 with multifunctional active sites[J]. Advanced Materials, 2018, 30(20).Doi: 10.1002/adma.201707105.
doi: 10.1002/adma.201707105 |
| [38] |
ZHANG L F, KE X X, OU G, et al. Defective MoS2 electrocatalyst for highly efficient hydrogen evolution through a simple ball-mill-ing method[J]. Science China Materials, 2017, 60(9):849-856.
doi: 10.1007/s40843-017-9086-9 |
| [39] | PU Z, LIU Q, ASIRI A M, et al. 3D macroporous MoS2 thin film:In situ hydrothermal preparation and application as a highly active hydrogen evolution electrocatalyst at all pH values[J]. Electrochi-mica Acta, 2015, 168:133-138. |
| [40] | CHEN I W P, HSIAO C H, HUANG J Y, et al. Highly efficient hy-drogen evolution from seawater by biofunctionalized exfoliated MoS2 quantum dot aerogel electrocatalysts that is superior to Pt[J]. ACS Applied Materials & Interfaces, 2019, 11(15):14159-14165. |
| [41] | HUANG X, LENG M, XIAO W, et al. Activating basal planes and S-terminated edges of MoS2 toward more efficient hydrogen evolution[J]. Advanced Functional Materials, 2017, 27(6):24-30. |
| [42] |
WANG J, LIU J, ZHANG B, et al. The mechanism of hydrogen ad-sorption on transition metal dichalcogenides as hydrogen evolution reaction catalyst[J]. Physical Chemistry Chemical Physics, 2017, 19(15):10125-10132.
doi: 10.1039/C7CP00636E |
| [43] |
PATTENGALE B, HUANG Y, YAN X, et al. Dynamic evolution and reversibility of single-atom Ni(Ⅱ) active site in 1T-MoS2 electro-catalysts for hydrogen evolution[J]. Nature Communications, 2020, 11(1):4114.
doi: 10.1038/s41467-020-17904-z |
| [44] |
LIU P, ZHU J, ZHANG J, et al. Active basal plane catalytic activity and conductivity in Zn doped MoS2 nanosheets for efficient hydrogen evolution[J]. Electrochimica Acta, 2018, 260:24-30.
doi: 10.1016/j.electacta.2017.11.080 |
| [45] |
HUANG Y C, SUN Y H, ZHENG X L, et al. Atomically engineering activation sites onto metallic 1T-MoS2 catalysts for enhanced elec-trochemical hydrogen evolution[J]. Nature Communications, 2019, 10(1):972-974.
doi: 10.1038/s41467-019-08865-z |
| [46] |
MA F, LIANG Y, ZHOU P, et al. One-step synjournal of Co-doped 1T-MoS2 nanosheets with efficient and stable HER activity in al-kaline solutions[J]. Materials Chemistry and Physics, 2020, 244. Doi: 10.1016/j.matchemphys.2020.122642.
doi: 10.1016/j.matchemphys.2020.122642 |
| [47] |
BIAN L Z, GAO W, SUN J M, et al. Phosphorus-doped MoS2 nano-sheets supported on carbon cloths as efficient hydrogen-generation electrocatalysts[J]. ChemCatChem, 2018, 10(7):1571-1577.
doi: 10.1002/cctc.v10.7 |
| [48] |
WANG Q, ZHAO Z L, DONG S, et al. Design of active nickel sin-gle-atom decorated MoS2 as a pH-universal catalyst for hydrogen evolution reaction[J]. Nano Energy, 2018, 53:458-467.
doi: 10.1016/j.nanoen.2018.09.003 |
| [49] |
ZHANG W, SUN Y F, LIU Q Y, et al. Vanadium and nitrogen co-doped CoP nanoleaf array as pH-universal electrocatalyst for effi-cient hydrogen evolution[J]. Journal of Alloys and Compounds, 2019, 791:1070-1078.
doi: 10.1016/j.jallcom.2019.03.380 |
| [50] |
CHEN J, JIA J, WEI Z Q, et al. Ni and N co-doped MoCx as efficient electrocatalysts for hydrogen evolution reaction at all-pH values[J]. International Journal of Hydrogen Energy, 2018, 43(31):14301-14309.
doi: 10.1016/j.ijhydene.2018.05.162 |
| [51] | SUN K, ZENG L, LIU S, et al. Design of basal plane active MoS2 through one-step nitrogen and phosphorus co-doping as an efficient pH-universal electrocatalyst for hydrogen evolution[J]. Nano En-ergy, 2019, 58:862-869. |
| [52] |
FAN A, ZHENG P, QIN C, et al. Few-layer MoS2 and Pt nanoparti-cles Co-anchored on MWCNTs for efficient hydrogen evolution over a wide pH range[J]. Electrochimica Acta, 2020, 358.Doi: 10.1016/j.electacta.2020.136927.
doi: 10.1016/j.electacta.2020.136927 |
| [53] |
FENG J X, WU J Q, TONG Y X, et al. Efficient hydrogen evolution on Cu nanodots-decorated Ni3S2 nanotubes by optimizing atomic hydrogen adsorption and desorption[J]. Journal of the American Chemical Society, 2018, 140(2):610-617.
doi: 10.1021/jacs.7b08521 |
| [54] |
FENG J X, TONG S Y, TONG Y X, et al. Pt-like hydrogen evo-lution electrocatalysis on PANI/CoP hybrid nanowires by weaken-ing the shackles of hydrogen ions on the surfaces of catalysts[J]. Journal of the American Chemical Society, 2018, 140(15):5118-5126.
doi: 10.1021/jacs.7b12968 |
| [55] |
GHUMAN K K, YADAV S, SINGH C V. Adsorption and dissocia-tion of H2O on monolayered MoS2 edges:Energetics and mechani-sm from ab initio simulations[J]. Journal of Physical Chemistry C, 2015, 119(12):6518-6529.
doi: 10.1021/jp510899m |
| [56] |
GONG M, WANG D Y, CHEN C C, et al. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction[J]. Nano Research, 2016, 9(1):28-46.
doi: 10.1007/s12274-015-0965-x |
| [57] |
GONG M, ZHOU W, TSAI M C, et al. Nanoscale nickel oxide/nic-kel heterostructures for active hydrogen evolution electrocataly-sis[J]. Nature Communications, 2014, 5(1).Doi: 10.1038/ncomms5695.
doi: 10.1038/ncomms5695 |
| [58] |
VIKRAMAN D, HUSSAIN S, TRUONG L, et al. Fabrication of MoS2/WSe2 heterostructures as electrocatalyst for enhanced hydro-gen evolution reaction[J]. Applied Surface Science, 2019, 480:611-620.
doi: 10.1016/j.apsusc.2019.02.236 |
| [59] |
HUANG L, YANG Y, ZHANG C, et al. A nanostructured MoO2/MoS2/MoP heterojunction electrocatalyst for the hydrogen evolu-tion reaction[J]. Nanotechnology, 2020, 31(22).Doi: 10.1088/1361-6528/ab767a.
doi: 10.1088/1361-6528/ab767a |
| [60] | ZHANG J, WANG T, LIU P, et al. Engineering water dissociation sites in MoS2 nanosheets for accelerated electrocatalytic hydrogen production[J]. Energy & Environmental Science, 2016, 9(9):2789-2793. |
| [61] |
PENG O, SHI R, WANG J, et al. Hierarchical heterostructured ni-ckle foam-supported Co3S4 nanorod arrays embellished with edge-exposed MoS2 nanoflakes for enhanced alkaline hydrogen evolution reaction[J]. Materials Today Energy, 2020, 18.Doi: 10.1016/j.mtener.2020.100513
doi: 10.1016/j.mtener.2020.100513 |
| [62] |
LIU S, LI B, MOHITE S V, et al. Ultrathin MoS2 nanosheets in situ grown on rich defective Ni0.96S as heterojunction bifunctional el-ectrocatalysts for alkaline water electrolysis[J]. International Journal of Hydrogen Energy, 2020, 45(55):29929-29937.
doi: 10.1016/j.ijhydene.2020.08.034 |
| [63] |
LIANG T T, LIU Y D, CHENG Y Z, et al. Scalable synjournal of a MoS2/Black phosphorus heterostructure for pH-universal hydro-gen evolution catalysis[J]. ChemCatChem, 2020, 12(10):2840-2848.
doi: 10.1002/cctc.v12.10 |
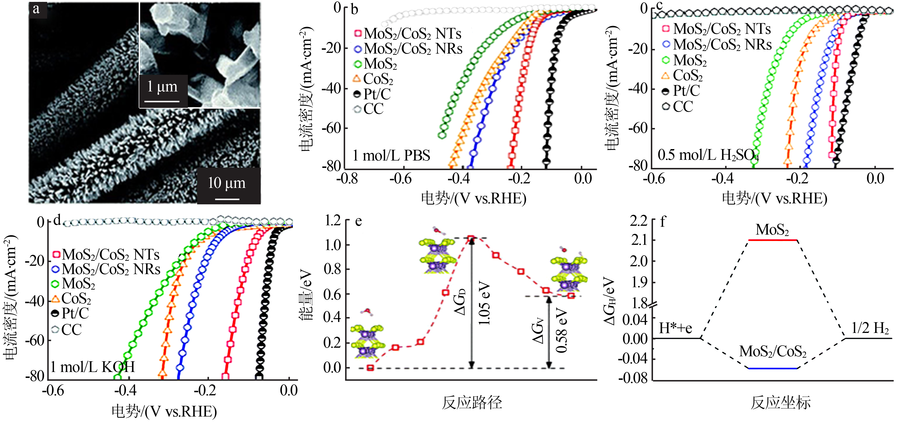
| [64] | TANG B, YU Z G, ZHANG Y, et al. Metal-organic framework-deri-ved hierarchical MoS2/CoS2 nanotube arrays as pH-universal elec-trocatalysts for efficient hydrogen evolution[J]. Journal of Materi-als Chemistry A, 2019, 7(21):13339-13346. |
| [1] | LI Yuhang, WANG Yinbin, WEI Qiang. Preparation of Fe2O3-Co3O4 heterojunction by molten salt method and its hydrogen evolution performance [J]. Inorganic Chemicals Industry, 2023, 55(8): 51-58. |
| [2] | WANG Wei,LIU Wei,WU Yang,YANG Shenshen. Research progress on molybdenum disulfide-based anode materials for lithium-ion batteries [J]. Inorganic Chemicals Industry, 2022, 54(10): 87-95. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||