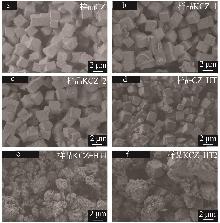
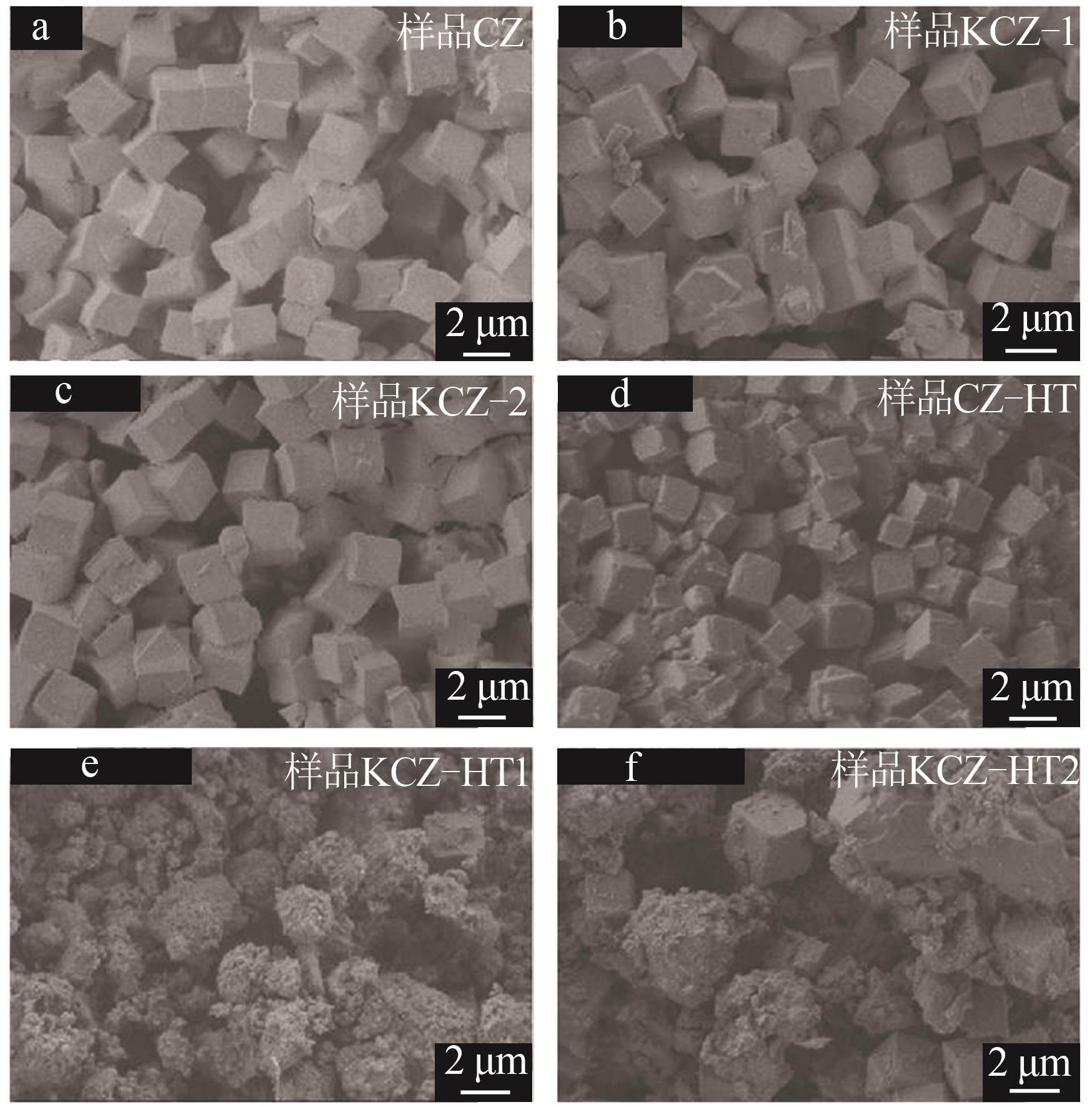
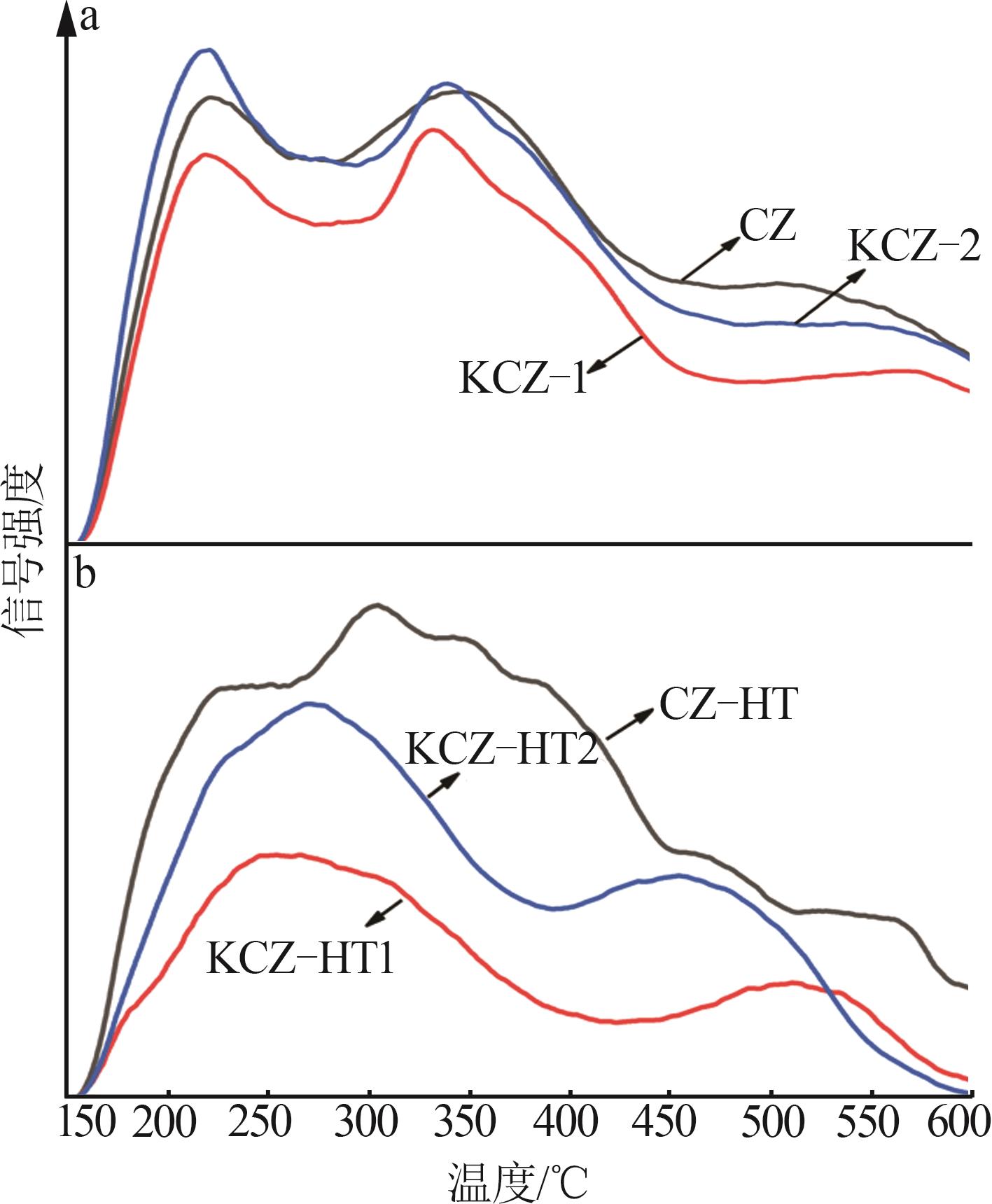
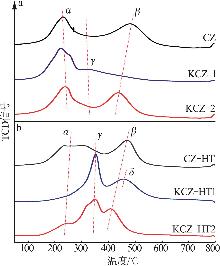
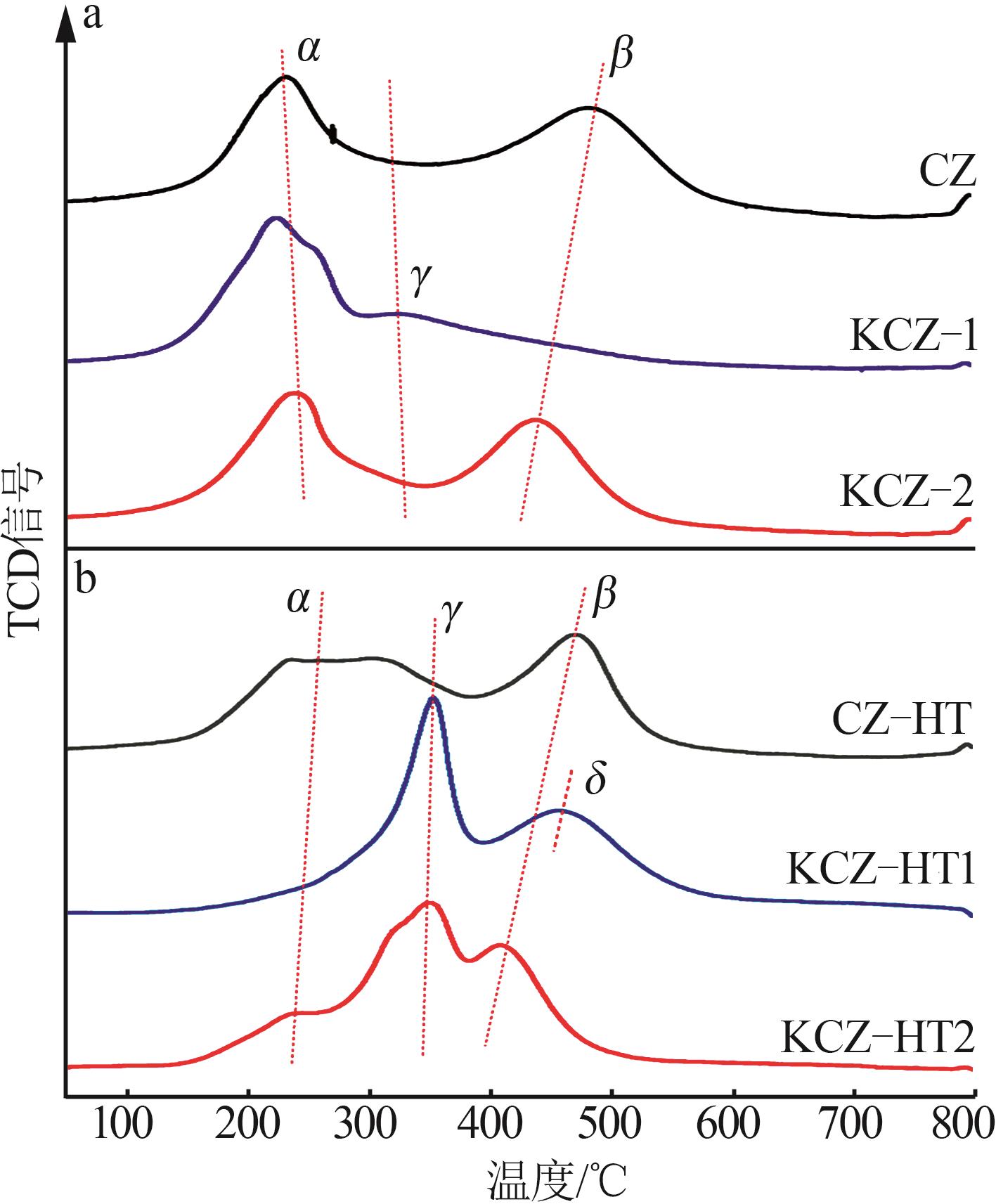
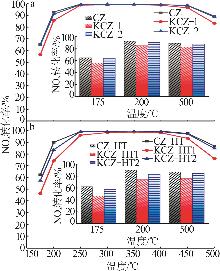
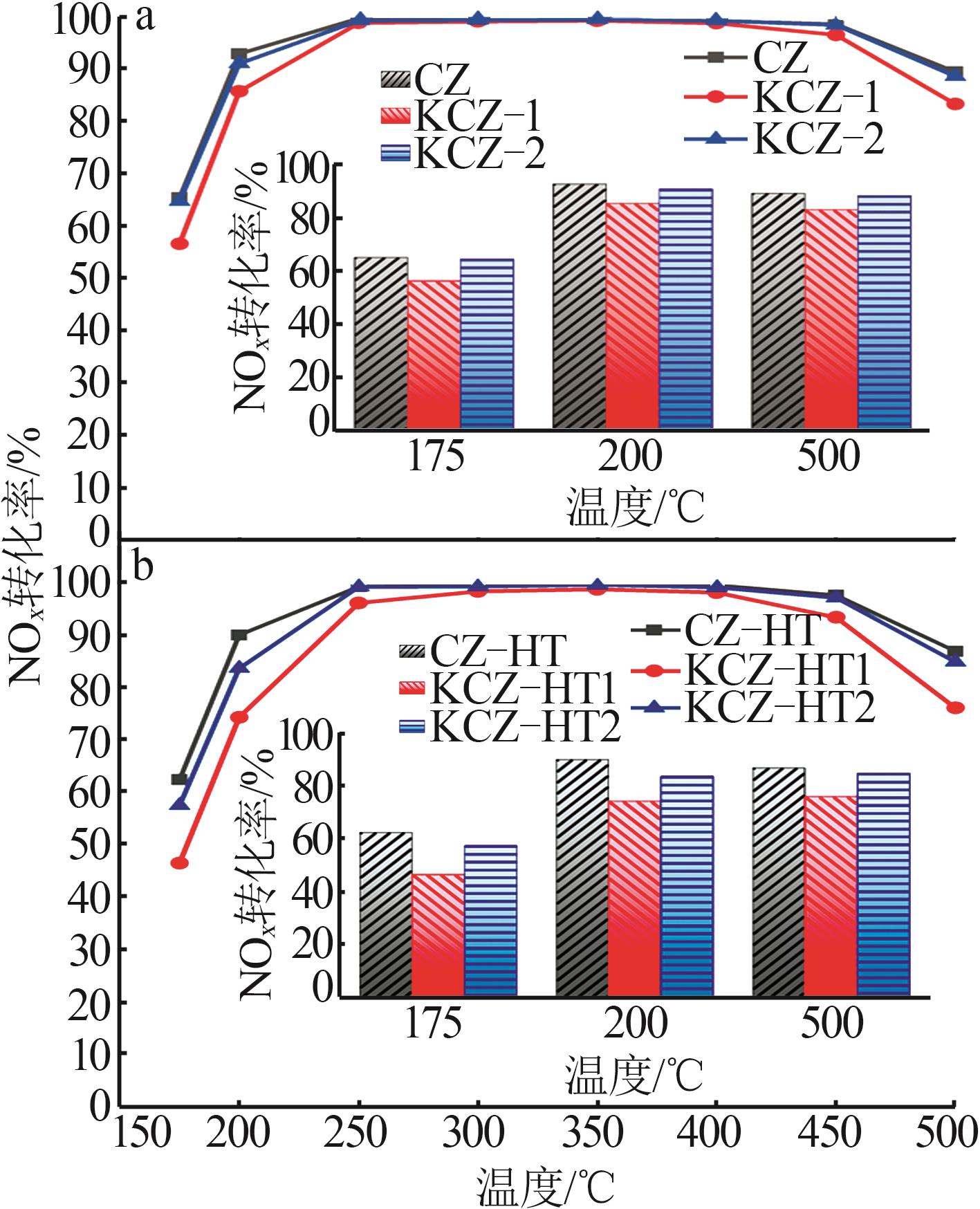
| [1] |
赖慧龙,于飞,杨冬霞,等.基于不同Cu-CHA催化剂方案的NH3-SCR性能及氨存储特性研究[J].无机盐工业,2024,56(12):159-166.
|
|
LAI Huilong, YU Fei, YANG Dongxia,et al.Research on NH3-SCR performance and ammonia storage characteristics based on different Cu-CHA catalyst schemes[J].Inorganic Chemicals Industry,2024,56(12):159-166.
|
| [2] |
谭仁俊,罗仕忠,王涛,等.不同元素掺杂锰基催化剂的NH3-SCR反应性能研究[J].无机盐工业,2023,55(7):115-121.
|
|
TAN Renjun, LUO Shizhong, WANG Tao,et al.Study on NH3-SCR performance of manganese based catalysts doped with different elements[J].Inorganic Chemicals Industry,2023,55(7):115-121.
|
| [3] |
SHI Zhiwei, PENG Qingguo, Jiaqiang E,et al.Mechanism,performance and modification methods for NH3-SCR catalysts:A revi- ew[J].Fuel,2023,331:125885.
|
| [4] |
LIN Qingjin, XU Shuhao, ZHAO Hongyan,et al.Highlights on key roles of Y on the hydrothermal stability at 900 ℃ of Cu/SSZ-39 for NH3-SCR[J].ACS Catalysis,2022,12(22):14026-14039.
|
| [5] |
SHAN Yulong, DU Jinpeng, ZHANG Yan,et al.Selective catalytic reduction of NO x with NH3:Opportunities and challenges of Cu-based small-pore zeolites[J].National Science Review,2021,8(10):nwab010.
|
| [6] |
KWAK J H, ZHU Haiyang, LEE J H,et al.Two different cationic positions in Cu-SSZ-13?[J].Chemical Communications,2012,48(39):4758-4760.
|
| [7] |
SONG J, WANG Yilin, WALTER E D,et al.Toward rational design of Cu/SSZ-13 selective catalytic reduction catalysts:Implications from atomic-level understanding of hydrothermal stability[J].ACS Catalysis,2017,7(12):8214-8227.
|
| [8] |
MA Lei, CHENG Yisun, CAVATAIO G,et al.Characterization of commercial Cu-SSZ-13 and Cu-SAPO-34 catalysts with hydrothermal treatment for NH3-SCR of NO x in diesel exhaust[J].Chemical Engineering Journal,2013,225:323-330.
|
| [9] |
李昊,邓志权,李国波,等.移动源分子筛脱硝催化剂研究进展[J].中国材料进展,2024,43(9):773-785,772.
|
|
LI Hao, DENG Zhiquan, LI Guobo,et al.Research progress of mobile source molecular sieve denitrification catalyst[J].Materials China,2024,43(9):773-785,772.
|
| [10] |
JIANG Han, GUAN Bin, PENG Xuesong,et al.Effect of sulfur poisoning on the performance and active sites of Cu/SSZ-13 catalyst[J].Chemical Engineering Science,2020,226:115855.
|
| [11] |
SONG Kunli, ZHAO Shuqi, LI Zhenguo,et al.Zinc and phosphorus poisoning tolerance of Cu-SSZ-13 and Ce-Cu-SSZ-13 in the catalytic reduction of nitrogen oxides[J].Journal of Colloid and Interface Science,2023,629:243-255.
|
| [12] |
GUO Jiangfeng, WANG Aiyong, LIN He.Effect of phosphorus poisoning on the hydrothermal stability of Cu/CHA and Cu/LTA towards NH3-SCR[J].Microporous and Mesoporous Materials,2022,346:112313.
|
| [13] |
JOUINI H, MEJRI I, MARTINEZ-ORTIGOSA J,et al.Alkali poisoning of Fe-Cu-ZSM-5 catalyst for the selective catalytic reduction of NO with NH3 [J].Research on Chemical Intermediates,2022,48(8):3415-3428.
|
| [14] |
FAN Chi, CHEN Zhen, PANG Lei,et al.Steam and alkali resistant Cu-SSZ-13 catalyst for the selective catalytic reduction of NO x in diesel exhaust[J].Chemical Engineering Journal,2018,334:344-354.
|
| [15] |
HAN Shuai, YE Qing, CHENG Shuiyuan,et al.Effect of the hydrothermal aging temperature and Cu/Al ratio on the hydrothermal stability of CuSSZ-13 catalysts for NH3-SCR[J].Catalysis Science & Technology,2017,7(3):703-717.
|
| [16] |
ZHANG Tao, LI Jianmei, LIU Jian,et al.High activity and wide temperature window of Fe-Cu-SSZ-13 in the selective catalytic reduction of NO with ammonia[J].AIChE Journal,2015,61(11):3825-3837.
|
| [17] |
WANG Can, WANG Chen, WANG Jun,et al.Effects of Na+ on Cu/SAPO-34 for ammonia selective catalytic reduction[J].Journal of Environmental Sciences,2018,70:20-28.
|
| [18] |
KIM Y J, LEE J K, MIN K M,et al.Hydrothermal stability of Cu-SSZ-13 for reducing NO x by NH3 [J].Journal of Catalysis,2014,311:447-457.
|
| [19] |
YONG Xin, ZHANG Cuijuan, WEI Miao,et al.Promotion of the performance of Cu-SSZ-13 for selective catalytic reduction of NO x by ammonia in the presence of SO2 during high temperature hydrothermal aging[J].Journal of Catalysis,2021,394:228- 235.
|
| [20] |
BULÁNEK R, WICHTERLOVÁ B, SOBALı́K Z,et al.Reducibility and oxidation activity of Cu ions in zeolites Effect of Cu ion coordination and zeolite framework composition[J].Applied Catalysis B:Environmental,2001,31(1):13-25.
|
| [21] |
ZHAO Zhenchao, YU Rui, ZHAO Rongrong,et al.Cu-exchanged Al-rich SSZ-13 zeolite from organotemplate-free synthesis as NH3-SCR catalyst:Effects of Na+ ions on the activity and hydrothermal stability[J].Applied Catalysis B:Environmental,2017,217:421-428.
|
| [22] |
FICKEL D W, LOBO R F.Copper coordination in Cu-SSZ-13 and Cu-SSZ-16 investigated by variable-temperature XRD[J].The Journal of Physical Chemistry C,2010,114(3):1633-1640.
|
| [23] |
HAN Jinfeng, LI Junyan, BAI Yang,et al.Cocrystallized zeolite interface boosting the hydrothermal stability of Cu-CHA/OFF-ERI catalysts[J].CCS Chemistry,2025,7(3):681-690.
|
| [24] |
SU Wenkang, LI Zhenguo, PENG Yue,et al.Correlation of the changes in the framework and active Cu sites for typical Cu/CHA zeolites(SSZ-13 and SAPO-34) during hydrothermal aging[J].Physical Chemistry Chemical Physics,2015,17(43):29142-29149.
|
| [25] |
GAO Feng, WALTER E D, WASHTON N M,et al.Synthesis and evaluation of Cu/SAPO-34 catalysts for NH3-SCR 2:Solid-state ion exchange and one-pot synthesis[J].Applied Catalysis B:Environmental,2015,162:501-514.
|
| [26] |
ZHENG Chaohe, ZHAO Haibo.The microscopic oxidation mechanism of NH3 on CuO(111):A first-principles study[J].Fuel Processing Technology,2021,213:106712.
|
 ), LÜ Liang1(
), LÜ Liang1( ), SUN Rui1, LAI Yineng1, YANG Lan1, WANG Ruifang1,2(
), SUN Rui1, LAI Yineng1, YANG Lan1, WANG Ruifang1,2( )
)