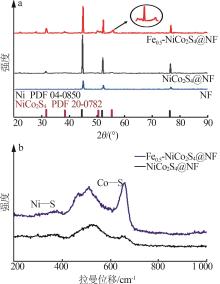
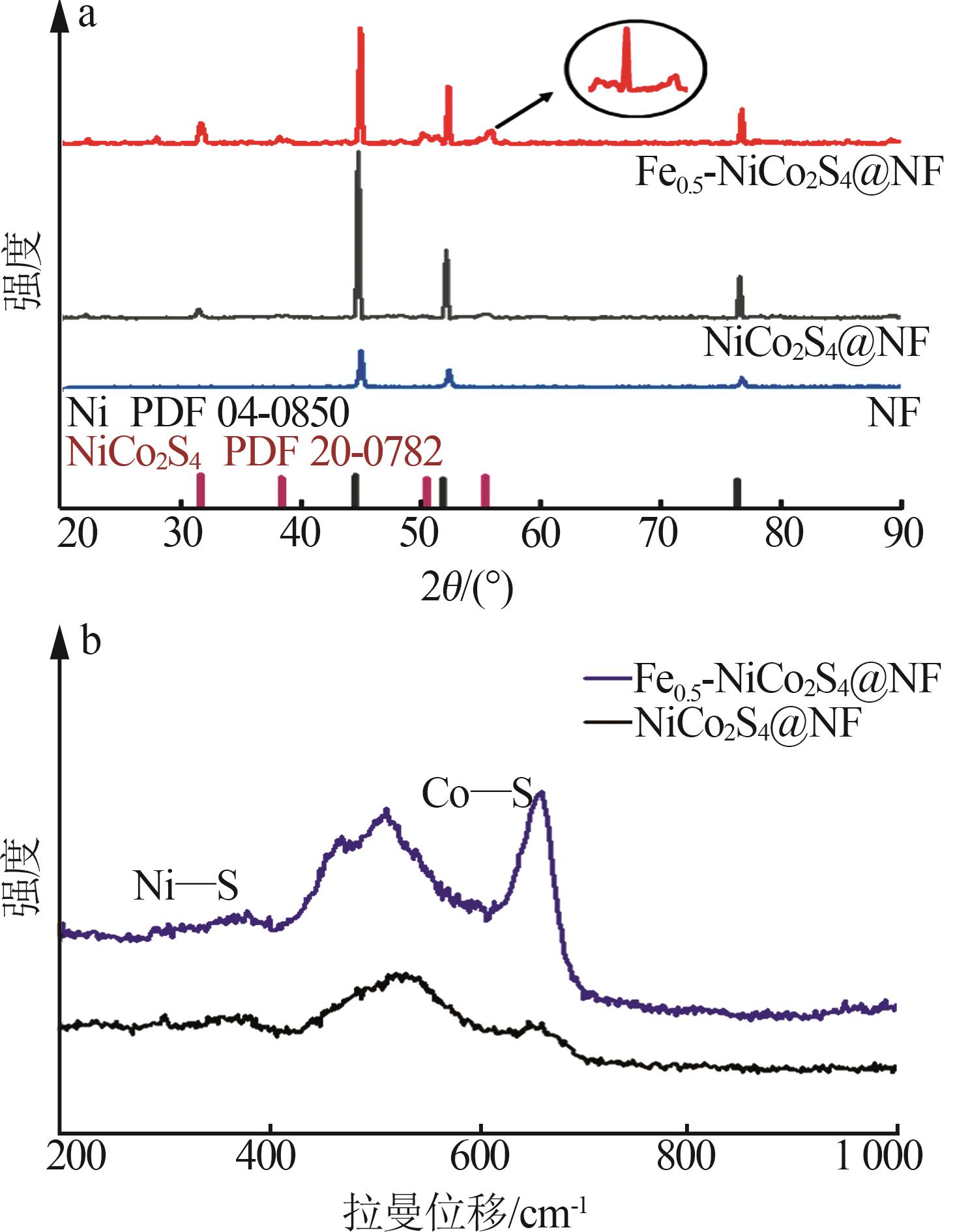
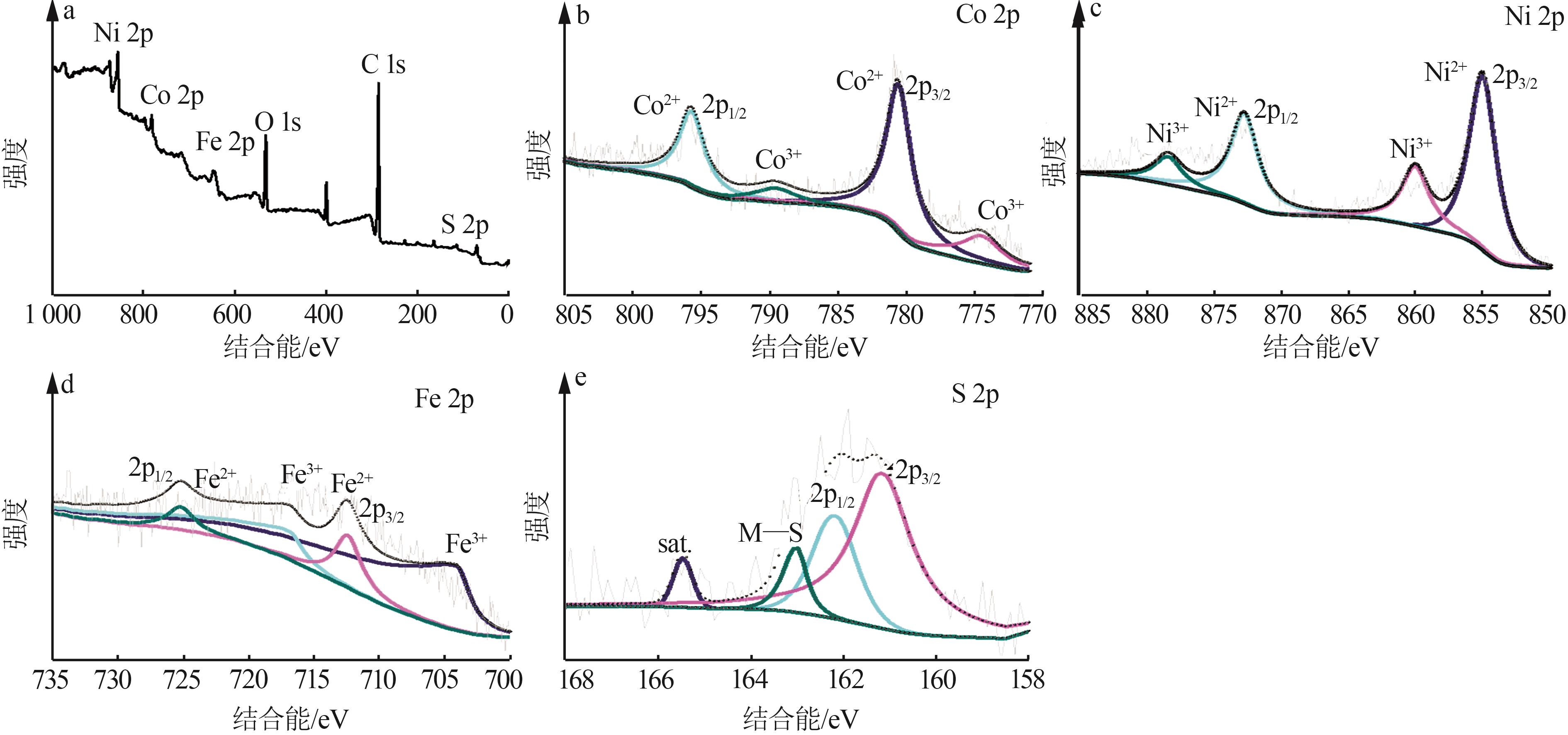
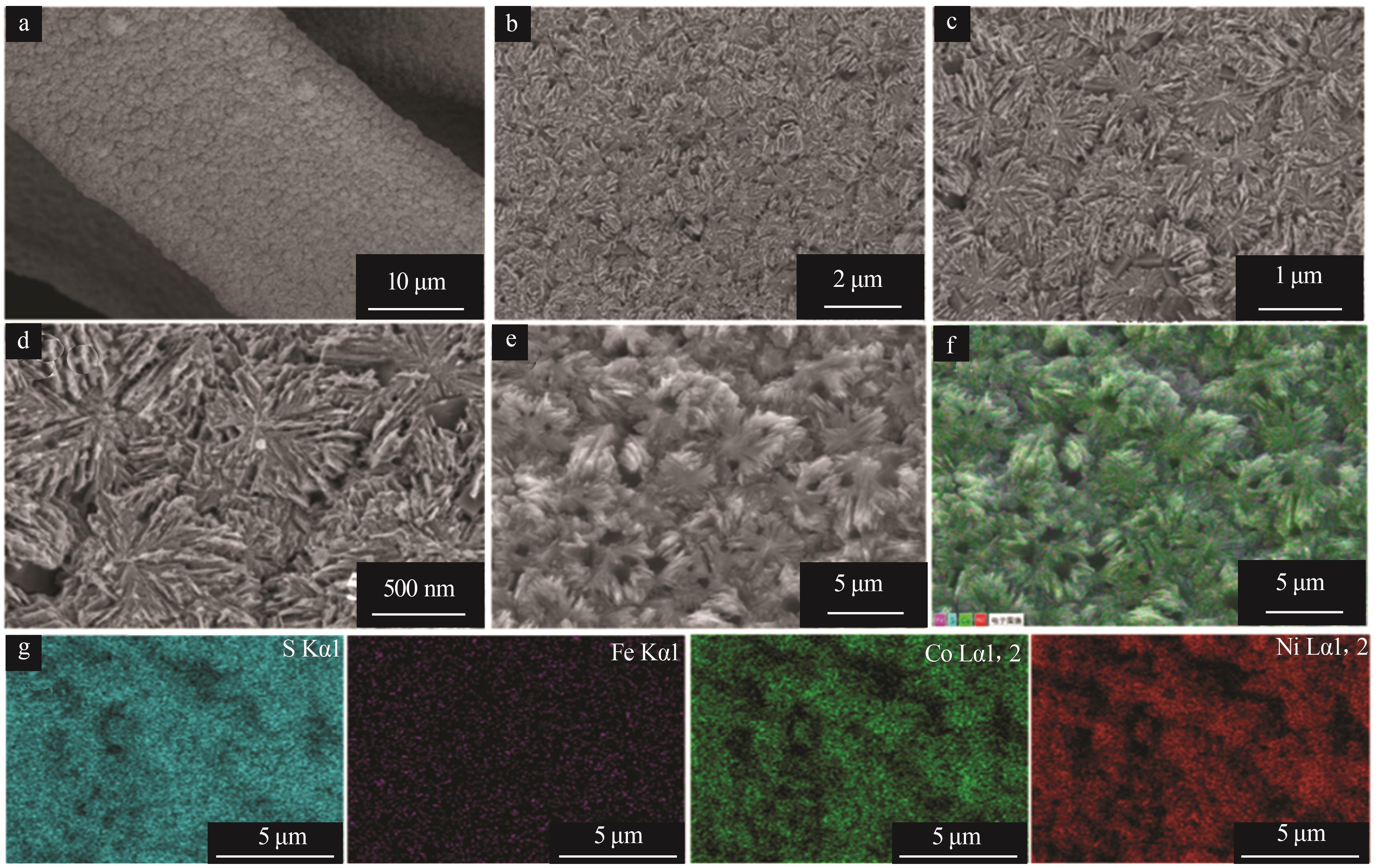
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (2): 130-137.doi: 10.19964/j.issn.1006-4990.2024-0182
• Catalytic Materials • Previous Articles Next Articles
Study on fabrication of nanoflower-like Fe y -NiCoS x @NF catalysts and their application in hydrogen evolution and oxygen evolution during seawater electrolysis
ZHANG Bao1,2( ), QUAN Kaidong2, WANG Yongfeng2, HAN Fei2, SHI Aiwen2, LIU Xin2, WANG Xiaomin1(
), QUAN Kaidong2, WANG Yongfeng2, HAN Fei2, SHI Aiwen2, LIU Xin2, WANG Xiaomin1( )
)
- 1.Taiyuan University of Technology School of Materials Science and Engineering,Taiyuan 030000,China
2.CNOOC(Shanxi) Precious Metals Co. ,LTD. ,Jinzhong 030600,China
-
Received:2024-04-02Online:2025-02-10Published:2024-06-03 -
Contact:WANG Xiaomin E-mail:1814009956@qq.com;wangxiaomin@tyut.edu.cn
CLC Number:
Cite this article
ZHANG Bao, QUAN Kaidong, WANG Yongfeng, HAN Fei, SHI Aiwen, LIU Xin, WANG Xiaomin. Study on fabrication of nanoflower-like Fe y -NiCoS x @NF catalysts and their application in hydrogen evolution and oxygen evolution during seawater electrolysis[J]. Inorganic Chemicals Industry, 2025, 57(2): 130-137.
share this article
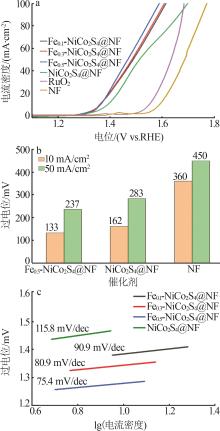
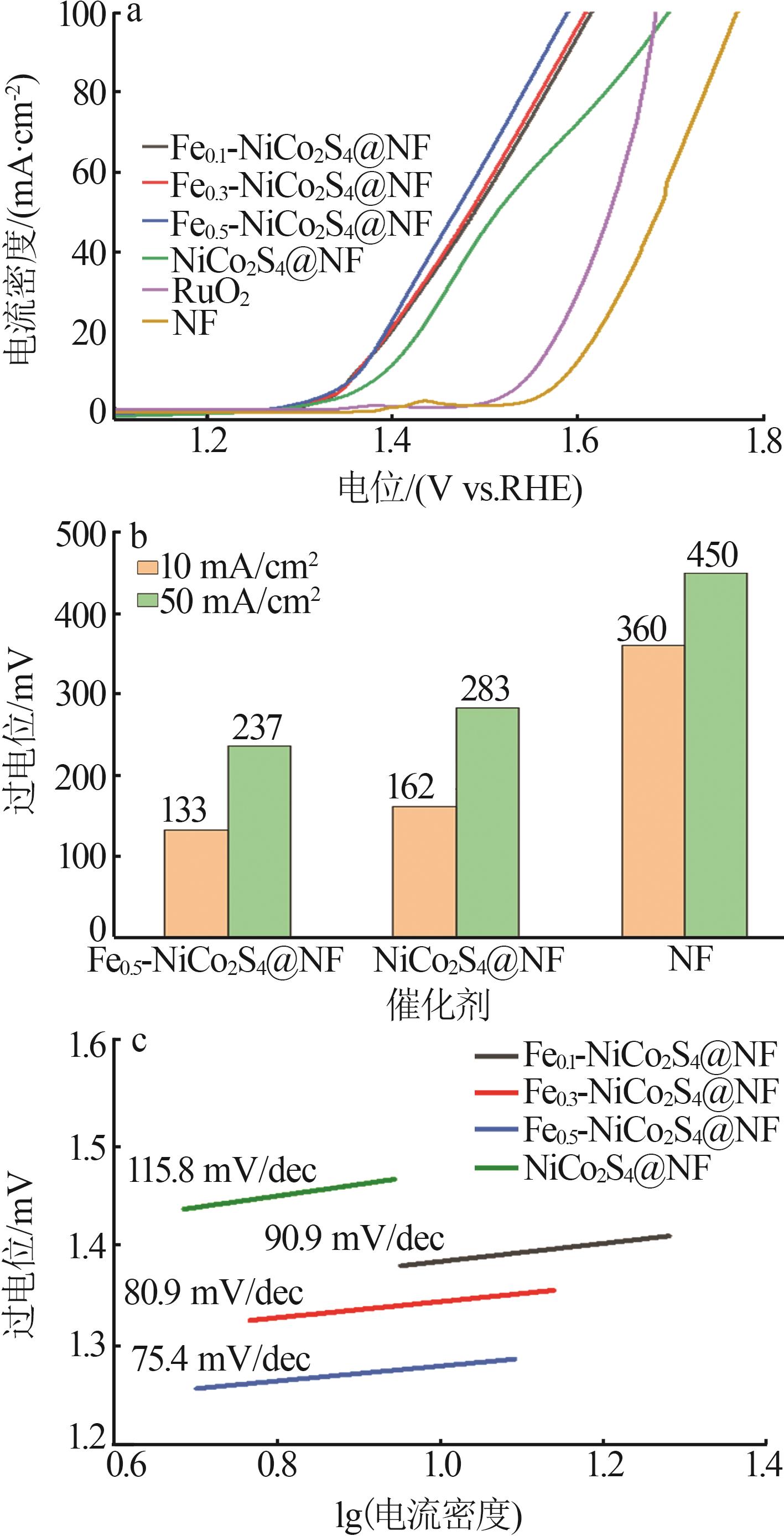
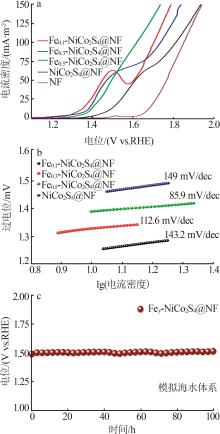
Table 1
Recently reported oxygen evolution performance of self-supported transition metal-based catalysts in 1.0 mol/L KOH"
| 催化剂材料 | 过电位(η10)/mV |
|---|---|
| Fe0.5-NiCoS x @NF*(本工作) | 133 |
| MgO/NCS-CC[ | 145 |
| Ni2P-Fe2P/NF[ | 218 |
| NiFe-LDH @NiCoP/NF[ | 220 |
| NiCo2S4@NF[ | 172 |
| Fe-NiCo2S4@NF[ | 181 |
| MoS2@CoFeLDH[ | 216 |
| A-NiFeV/NF[ | 234 |
| NiS/NiCo2S4[ | 259 |
| NiFeRu-LDH/NF[ | 225 |
| 1 | 裴洪昌,岳茂文,刘建路,等.海水综合开发与高效利用研究进展[J].无机盐工业,2024,56(2):21-29. |
| PEI Hongchang, YUE Maowen, LIU Jianlu,et al.Research progress of comprehensive development and efficient utilization of seawater[J].Inorganic Chemicals Industry,2024,56(2):21-29. | |
| 2 | 陈星亮,范文娟,常会,等.Fe3+协同Ni基金属-有机框架提升电催化析氧反应活性的研究[J].无机盐工业,2024,56(2):152- 158. |
| CHEN Xingliang, FAN Wenjuan, CHANG Hui,et al.Study on collaborative strategy between Fe3+ and Ni-based metal-organic frameworks for boosting electrocatalytic oxygen evolution[J].Inorganic Chemicals Industry,2024,56(2):152-158. | |
| 3 | 关亚峰,朱胜利,李朝阳,等.耐蚀镍基催化剂的制备及电解海水性能的研究[J].功能材料,2023,54(9):9001-9006,9021. |
| GUAN Yafeng, ZHU Shengli, LI Zhaoyang,et al.Preparation of corrosion resistant nickel-based materials and study on the performance for seawater electrolysis[J].Journal of Functional Materials,2023,54(9):9001-9006,9021. | |
| 4 | 武讨龙,张胜江,刘津洋,等.双金属有机框架材料的研究进展[J].无机盐工业,2023,55(6):8-17. |
| WU Taolong, ZHANG Shengjiang, LIU Jinyang,et al.Research progress of bimetallic-organic frameworks materials[J].Inorganic Chemicals Industry,2023,55(6):8-17. | |
| 5 | GUO Peifang, LIU Da, WU Renbing.Recent progress in design strategy of anode for seawater electrolysis[J].Small Structures,2023,4(12):2300192. |
| 6 | ZHANG Yuanyuan, FU Qiang, SONG Bo,et al.Regulation strategy of transition metal oxide-based electrocatalysts for enhanced oxygen evolution reaction[J].Accounts of Materials Research,2022,3(10):1088-1100. |
| 7 | ZHANG Liyue, XU Qiucheng, ZHAO Rukai,et al.Fe-doped and sulfur-enriched Ni3S2 nanowires with enhanced reaction kinetics for boosting water oxidation[J].Green Chemical Engineering,2022,3(4):367-373. |
| 8 | MA Tengfei, XU Wenwen, LI Boran,et al.The critical role of additive sulfate for stable alkaline seawater oxidation on nickel-based electrodes[J].Angewandte Chemie,2021,60(42):22740-22744. |
| 9 | 周旋,李梦锐,陈一尘,等.镍基磷化物复合材料在催化电解水析氢性能提升方面的研究进展[J].无机盐工业,2024,56(4):8-15,33. |
| ZHOU Xuan, LI Mengrui, CHEN Yichen,et al.Research progress of nickel-based phosphide composites in improving of catalytic water electrolysis for hydrogen evolution performance[J].Inorganic Chemicals Industry,2024,56(4):8-15,33. | |
| 10 | LUO Wei, YU Yanli, WU Yucheng,et al.Realizing efficient oxygen evolution at low overpotential via dopant-induced interfacial coupling enhancement effect[J].Applied Catalysis B:Environmental,2023,336:122928. |
| 11 | LIU Jin, MENG Xin, XIE Jiahao,et al.Dual active sites engineering on sea urchin-like CoNiS hollow nanosphere for stabilizing oxygen electrocatalysis via a template-free vulcanization strategy[J].Advanced Functional Materials,2023,33(22):2300579. |
| 12 | LIU Yanyan, ZHOU Limin, LIU Shuling,et al.Fe,N-inducing interfacial electron redistribution in NiCo spinel on biomass-derived carbon for bi-functional oxygen conversion[J].Angewandte Chemie International Edition,2024,63(16):e202319983. |
| 13 | LI Haoquan, CHEN Long, JIN Pengfei,et al.NiCo2S4 microspheres grown on N,S co-doped reduced graphene oxide as an efficient bifunctional electrocatalyst for overall water splitting in alkaline and neutral pH[J].Nano Research,2022,15(2):950- 958. |
| 14 | SONG Lili, ZHANG Xiaoyun, ZHU Shifan,et al.Transition metal (Fe,Ni,and Zn) doping-induced modulation of geometric and electronic structures to optimize the potential-determining step of MnCo2O4 for oxygen evolution reaction[J].Science China Materials,2022,65(10):2871-2878. |
| 15 | FENG Xueting, JIAO Qingze, CUI Huiru,et al.One-pot synthesis of NiCo2S4 hollow spheres via sequential ion-exchange as an enhanced oxygen bifunctional electrocatalyst in alkaline solution[J].ACS Applied Materials & Interfaces,2018,10(35):29521-29531. |
| 16 | FENG Xueting, JIAO Qingze, CHEN Wenxing,et al.Cactus-like NiCo2S4@NiFe LDH hollow spheres as an effective oxygen bifunctional electrocatalyst in alkaline solution[J].Applied Catalysis B:Environmental,2021,286:119869. |
| 17 | SONG Yanyan, SUN Mingzi, ZHANG Shucong,et al.Alleviating the work function of vein-like Co X P by Cr doping for enhanced seawater electrolysis[J].Advanced Functional Materials,2023,33(30):2214081. |
| 18 | GHOSH S, DASGUPTA B, KALRA S,et al.Evolution of carbonate-intercalated γ-NiOOH from a molecularly derived nickel sulfide(pre)catalyst for efficient water and selective organic oxidation[J].Small,2023,19(16):2206679. |
| 19 | WANG Peng, HAN Xiao, BAI Ping,et al.Utilizing an electron redistribution strategy to inhibit the leaching of sulfur from CeO2/NiCo2S4 heterostructure for high-efficiency oxygen evolution[J].Applied Catalysis B:Environment and Energy,2024,344:123659. |
| 20 | GAO Hong, ZHOU Tengfei, ZHENG Yang,et al.CoS quantum dot nanoclusters for high-energy potassium-ion batteries[J].Advanced Functional Materials,2017,27(43):1702634. |
| 21 | LV Jinlong, LIANG Tongxiang, YANG Meng,et al.Performance comparison of NiCo2O4 and NiCo2S4 formed on Ni foam for supercapacitor[J].Composites Part B:Engineering,2017,123:28- 33. |
| 22 | QIAN Lihu, DONG Weiwei, LI Haibin,et al.Fe-doped NiCo2S4 catalyst derived from ZIF-67 towards efficient hydrogen evolution reaction[J].International Journal of Hydrogen Energy,2022,47(14):8820-8828. |
| 23 | VEERAMANI V, MADHU R, CHEN Shenming,et al.NiCo2O4-decorated porous carbon nanosheets for high-performance supercapacitors[J].Electrochimica Acta,2017,247:288-295. |
| 24 | PU Jun, WANG Tingting, WANG Haiyang,et al.Direct growth of NiCo2S4 nanotube arrays on nickel foam as high-performance bin-der-free electrodes for supercapacitors[J].ChemPlusChem,2014,79(4):577-583. |
| 25 | MA Dandan, LV Minglian, SHEN Yu,et al.Fabrication of porous mesh-like FeCo2S4 nanosheet arrays on Ni foam for high performance all solid-state supercapacitors and water splitting[J].ChemistrySelect,2019,4(6):1879-1889. |
| 26 | LI Wei, WU Shuangshuang, ZHANG Haoran,et al.Enhanced biological photosynthetic efficiency using light-harvesting engineering with dual-emissive carbon dots[J].Advanced Functional Materials,2018,28(44):1804004. |
| 27 | SONG Zhongxin, WANG K C, SUN Qian,et al.High-performance ammonium cobalt phosphate nanosheet electrocatalyst for alkaline saline water oxidation[J].Advanced Science,2021,8(14):2100498. |
| 28 | JIN Mixue, PU Yulu, WANG Zijuan,et al.Facile synthesis of 3D NiCoP@NiCoPO x core-shell nanostructures with boosted catalytic activity toward oxygen evolution reaction[J].ACS Applied Energy Materials,2019,2(6):4188-4194. |
| 29 | CHEN Weizhe, ZHANG Meng, LIU Yang,et al.RETRACTED:Super-hydrophilic MgO/NiCo2S4 heterostructure for high-efficient oxygen evolution reaction in neutral electrolytes[J].Applied Catalysis B:Environmental,2022,312:121432. |
| 30 | WU Libo, YU Luo, ZHANG Fanghao,et al.Heterogeneous bimetallic phosphide Ni2P-Fe2P as an efficient bifunctional catalyst for water/seawater splitting[J].Advanced Functional Materials,2021,31(1):2006484. |
| 31 | ZHANG Haojie, LI Xiaopeng, HÄHNEL A,et al.Bifunctional heterostructure assembly of NiFe LDH nanosheets on NiCoP nano-wires for highly efficient and stable overall water splitting[J].Advanced Functional Materials,2018,28(14):1706847. |
| 32 | SUN Yingying, ZHANG Xinyu, TANG Jianfang,et al.Amorphous oxysulfide reconstructed from spinel NiCo2S4 for efficient water oxidation[J].Small,2023,19(27):2207965. |
| 33 | XU Yang, CHENG Jun, DING Liwei,et al.Interfacial engineering for promoting charge transfer in MoS2/CoFeLDH heterostructure electrodes for overall water splitting[J].International Journal of Hydrogen Energy,2024,49:897-906. |
| 34 | YANG Hua, GE Lihong, GUAN Jiexin,et al.Synergistic engineering of heteroatom doping and heterointerface construction in V-doped Ni(OH)2/FeOOH to boost both oxygen evolution and urea oxidation reactions[J].Journal of Colloid and Interface Science,2024,653:721-729. |
| 35 | WAN Zhenwei, ZHANG Yueqi, REN Qinglin,et al.Interface engineering of NiS/NiCo2S4 heterostructure with charge redistribution for boosting overall water splitting[J].Journal of Colloid and Interface Science,2024,653:795-806. |
| 36 | CHEN Guangbo, WANG Tao, ZHANG Jian,et al.Accelerated hydrogen evolution kinetics on NiFe-layered double hydroxide electrocatalysts by tailoring water dissociation active sites[J].Advanced Materials,2018,30(10):1706279. |
| [1] | LUO Chengling, FAN Xiaofan. Research progress of microstructure-regulated catalysts for urea oxidation reactions [J]. Inorganic Chemicals Industry, 2025, 57(2): 26-35. |
| [2] | CHEN Xingliang, FAN Wenjuan, CHANG Hui, HUANG Haiping, JIANG Zhiqiang. Study on collaborative strategy between Fe3+ and Ni-based metal-organic frameworks for boosting electrocatalytic oxygen evolution [J]. Inorganic Chemicals Industry, 2024, 56(2): 152-158. |
| [3] | YUAN Shuai, FANG Yangfei, YANG Xiangguang, ZHANG Yibo. Study on synthesis of rare earth-doped CeO2 and its CMP properties [J]. Inorganic Chemicals Industry, 2024, 56(12): 35-41. |
| [4] | CHEN Junxue, MO Jianxin, ZHOU Zhiyu, LI Zhonglin, WANG Ding, LI Yuping, HU Yongjun, JIANG Xuexian, LI Yibing. Study on synthesis of NaFe x Cr y (SO4)2(OH)6 and their electrochemical properties [J]. Inorganic Chemicals Industry, 2023, 55(8): 71-76. |
| [5] | TANG Hu,LIU Fang. Study on preparation of molybdenum cobalt binary sulfide and its performance in water splitting [J]. Inorganic Chemicals Industry, 2022, 54(2): 65-71. |
| [6] | Zhang Jie,Zhao Mengjie,Cui Yingqi,Li Chenggang,Gao Jinhai. Research progress in effect of doping on properties of CeO2-based electrolyte materials [J]. Inorganic Chemicals Industry, 2020, 52(5): 1-5. |
| [7] | Long Junxi,Xu Xuetang,Wang Fan. Preparation of porous CoFe LDH nanosheets electrocatalyst by low-temperature co-precipitation method [J]. Inorganic Chemicals Industry, 2020, 52(12): 98-103. |
| [8] | YANG Lei-Lei, LI Fa-Qiang, JIA Guo-Feng, PENG Zheng-Jun, ZHU Chao-Liang, ZHU Hong. Research progress in cathode materials for rechargeable magnesium battery [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(2): 6-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||