Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (11): 43-46.doi: 10.11962/1006-4990.2019-0619
• Research & Development • Previous Articles Next Articles
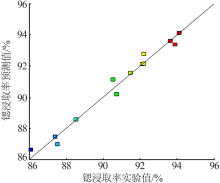
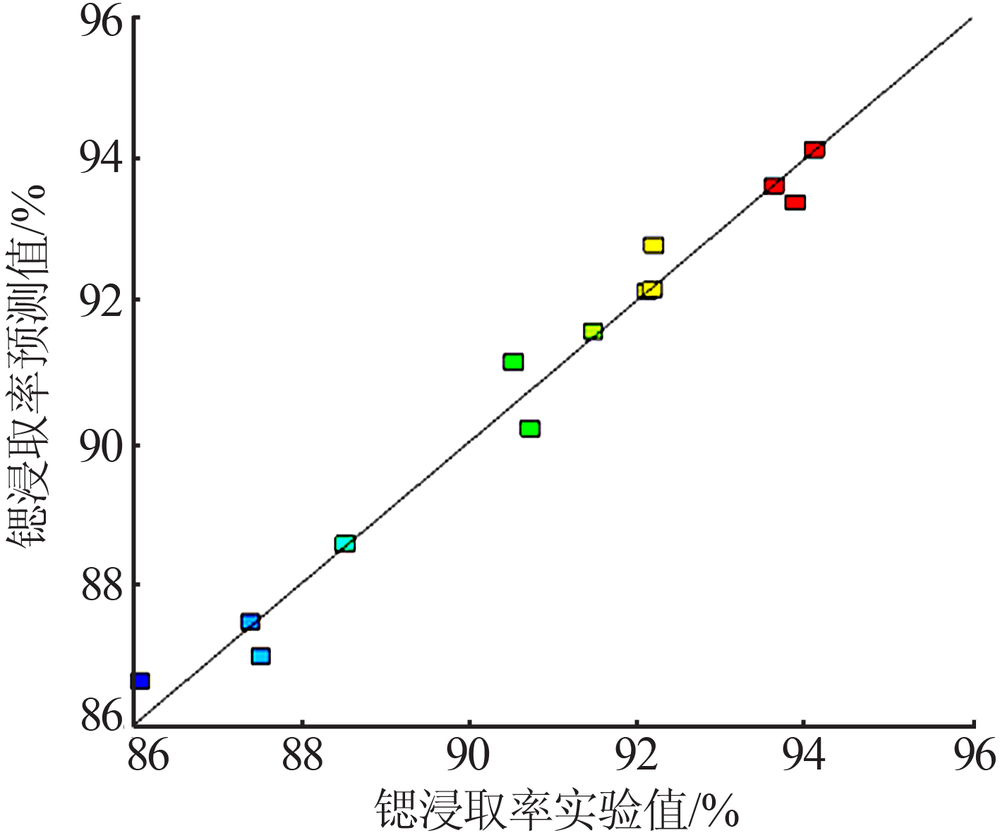
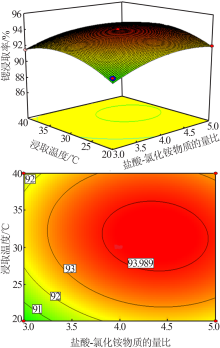
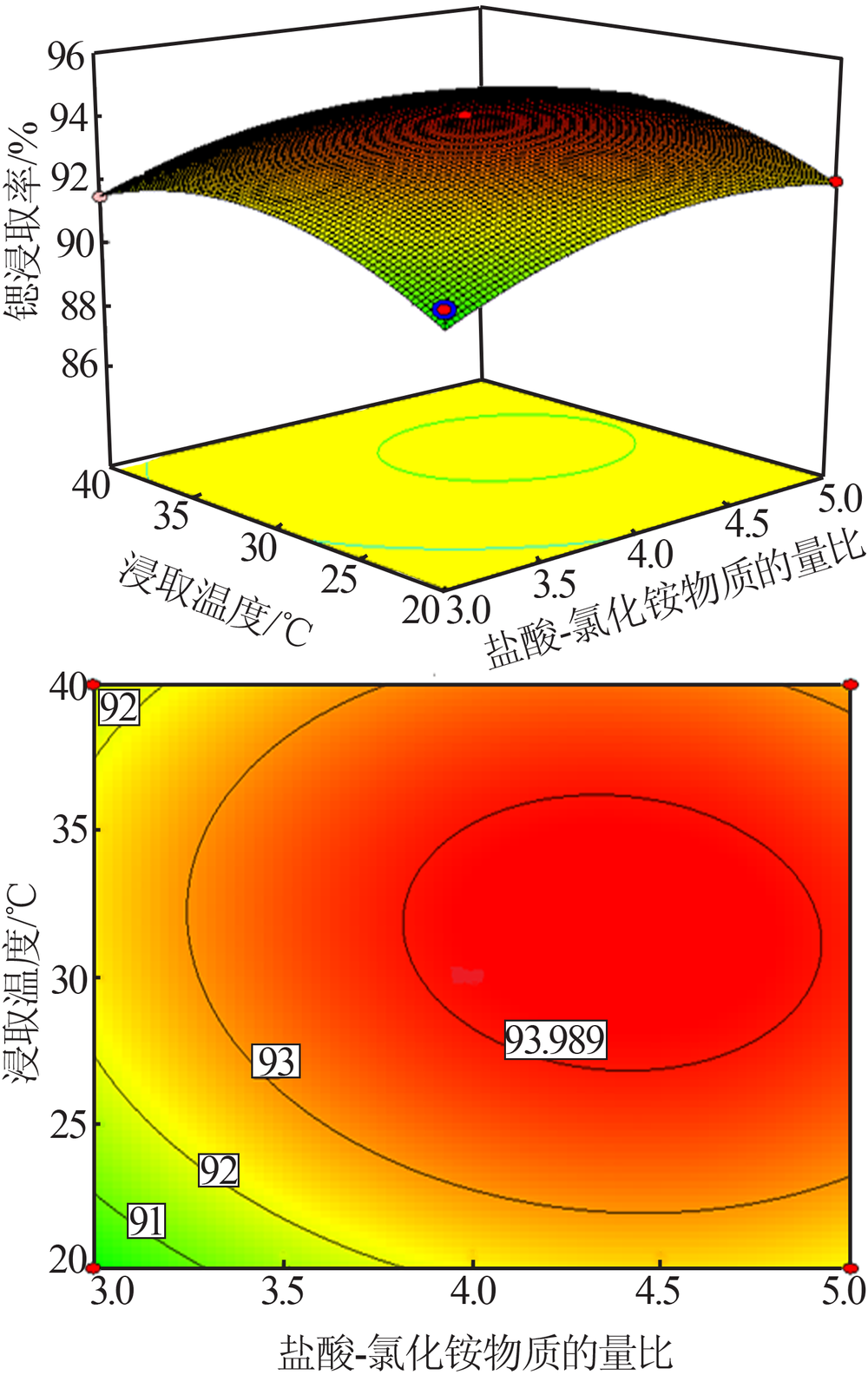
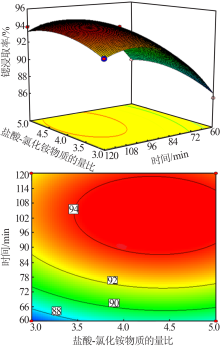
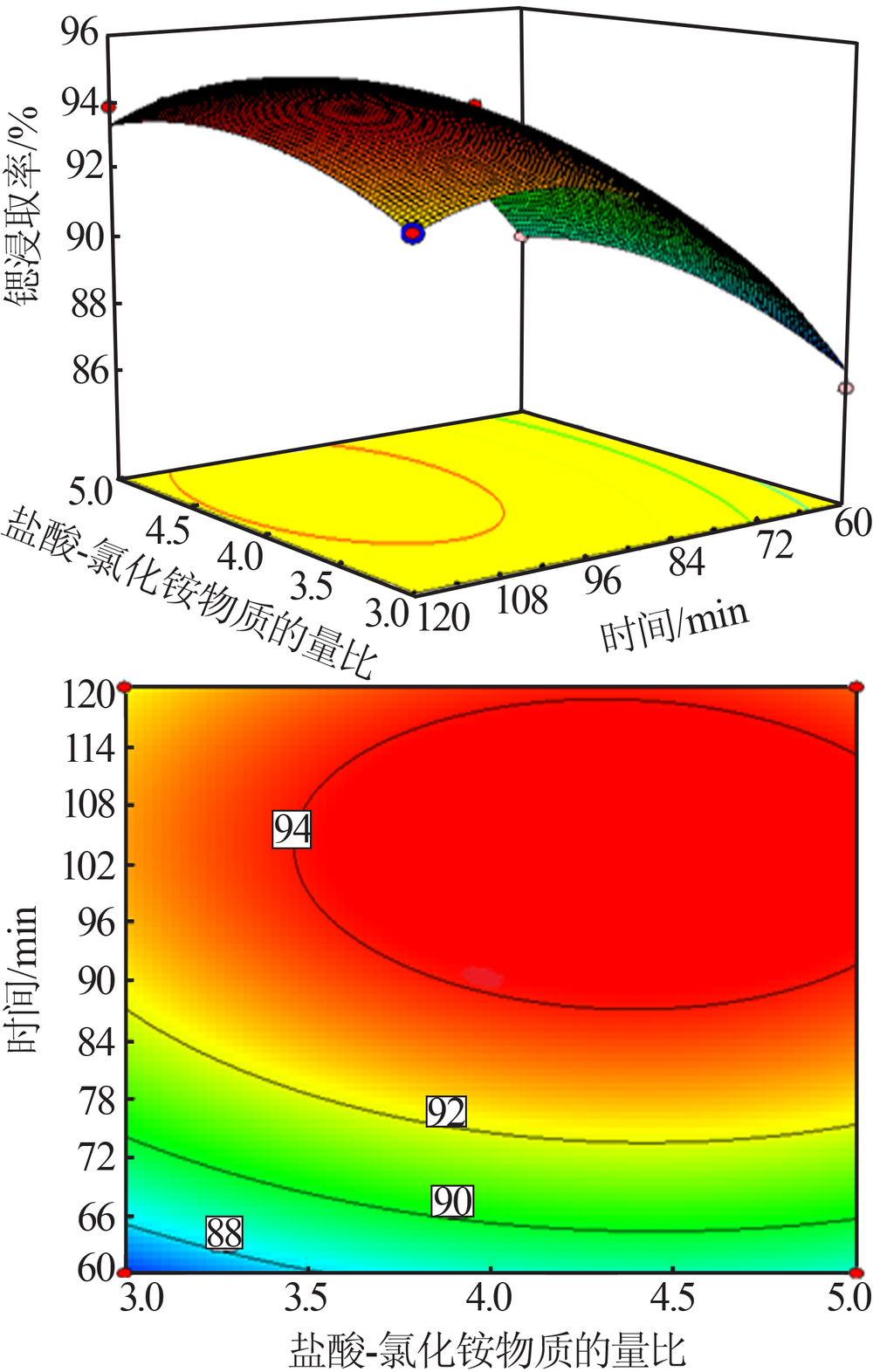
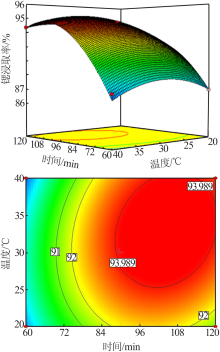
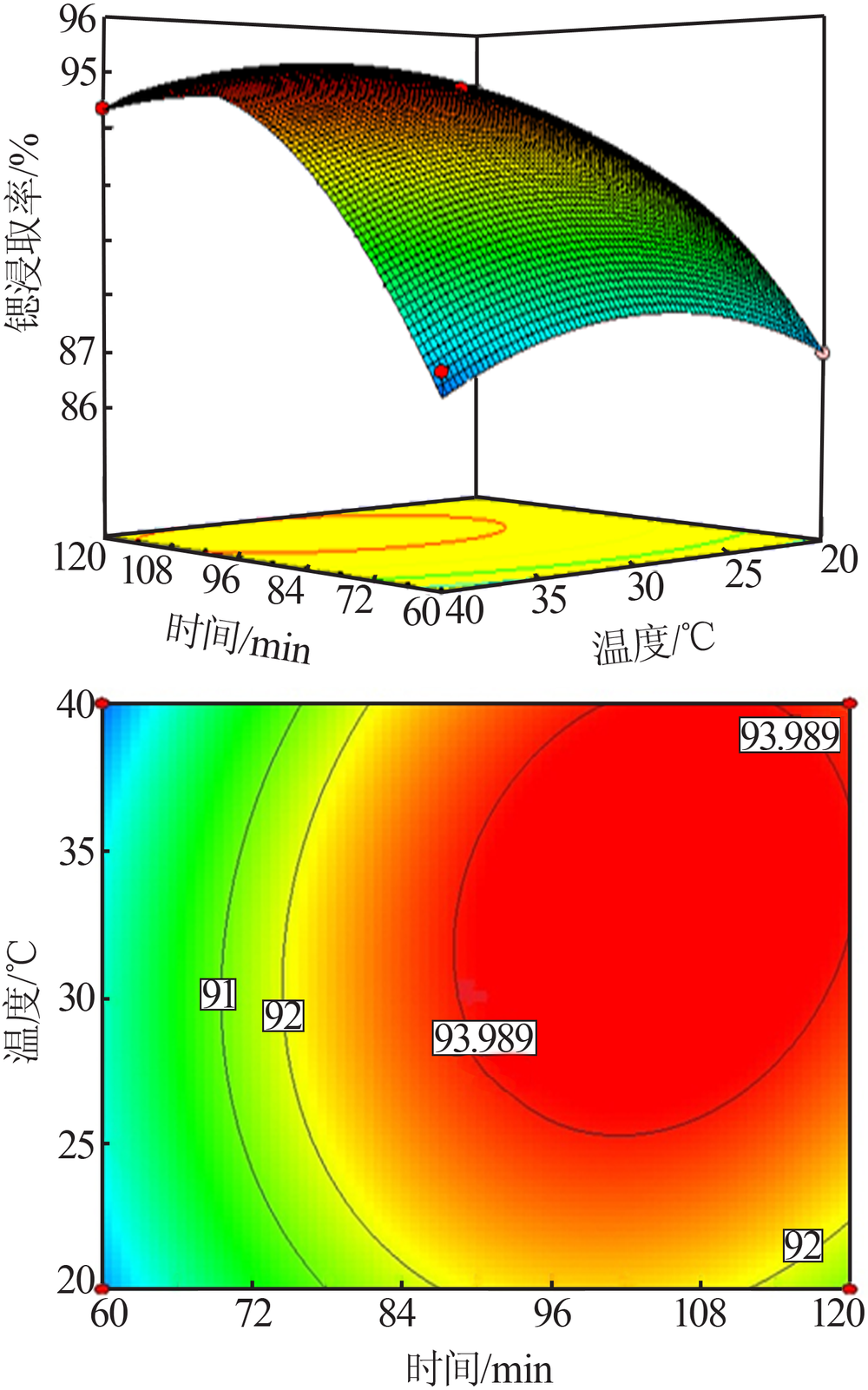
Study on leaching process of strontium carbonate residue with hydrochloric acid and ammonium chloride
Jin Jianhua( ),Zhou Chengcai,Feng Zhanming
),Zhou Chengcai,Feng Zhanming
- School of Chemistry and Chemical Engineering,Qinghai University for Nationalities,Xining 810007,China