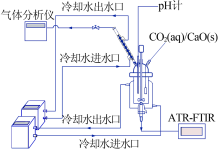
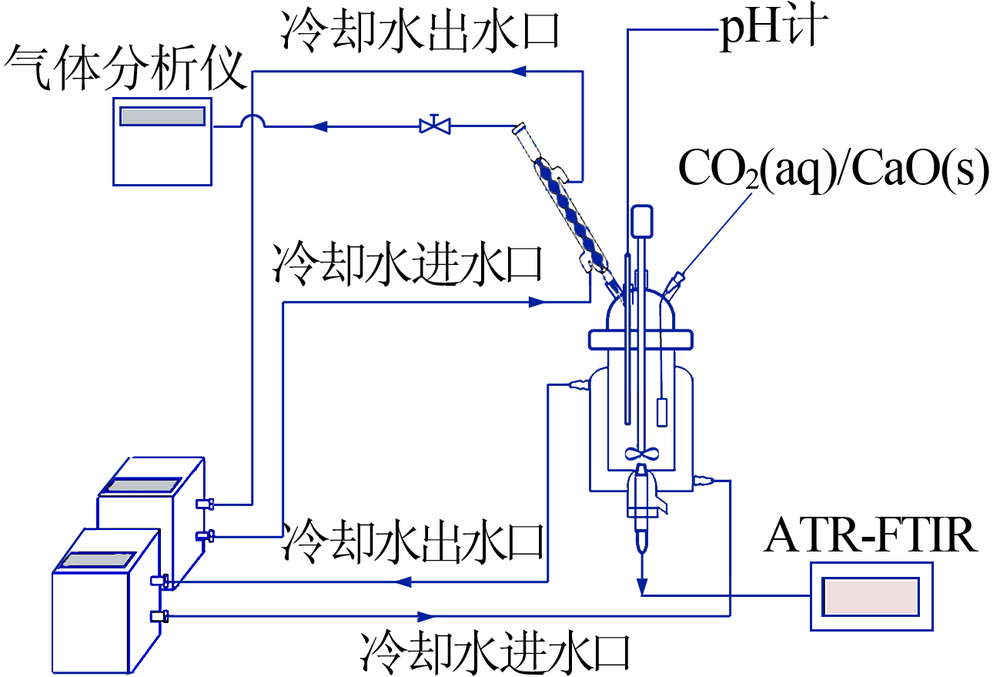
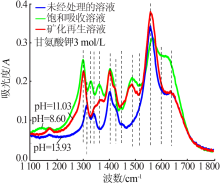
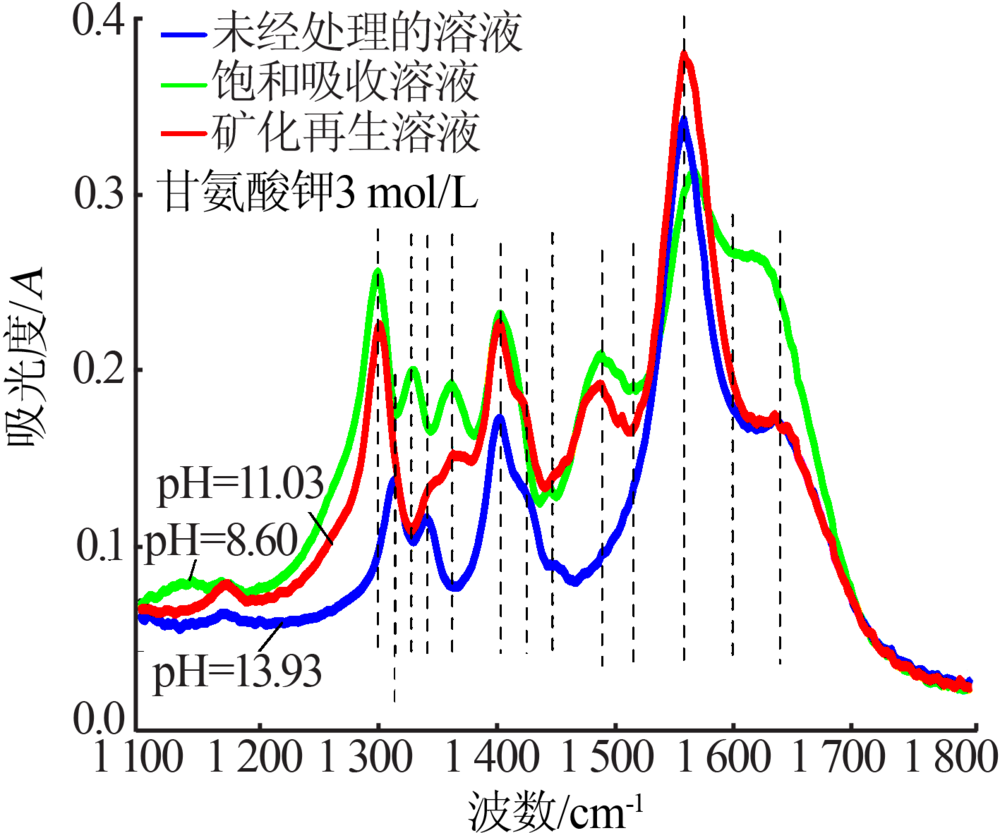
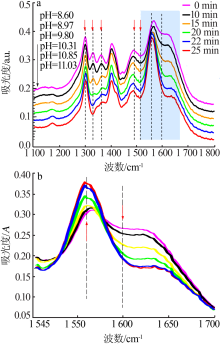
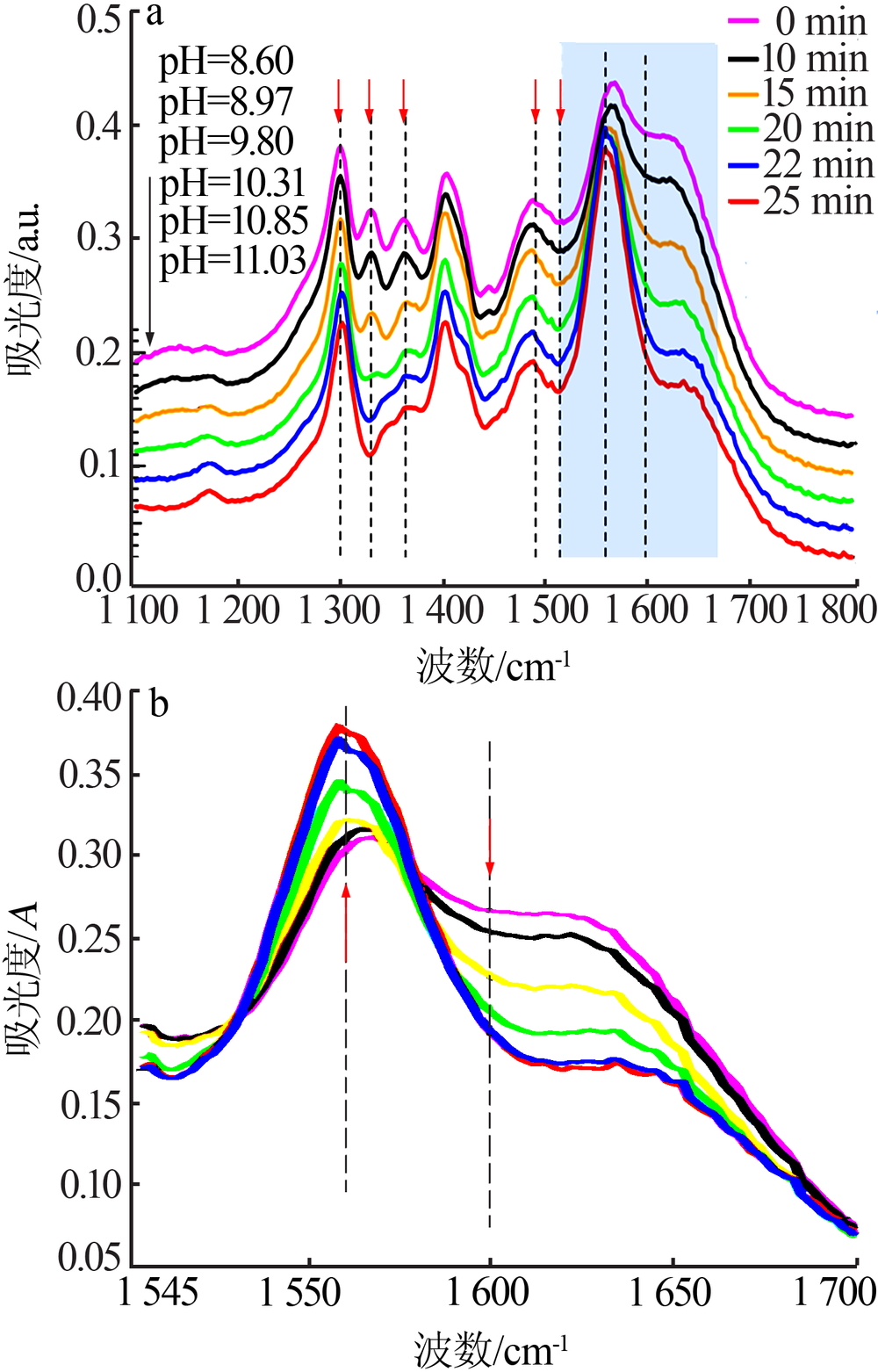
| [1] |
Matter J M, Stute M, Snaebjornsdottir S O, et al. Rapid carbon mi-neralization for permanent disposal of anthropogenic carbon dioxide emissions[J]. Science, 2016,352(6291):1312-1314.
doi: 10.1126/science.aad8132
pmid: 27284192
|
| [2] |
Rochelle G T. Amine scrubbing for CO2 capture[J]. Science, 2009,325(5948):1652-1654.
doi: 10.1126/science.1176731
pmid: 19779188
|
| [3] |
Zevenhoevn R, Fagerlund J, Songok J K. CO2 mineral sequestration:Developments toward large-scale application[J].Greenhouse Gases:Science and Technology, 2011(1):48-57.
|
| [4] |
Sanna A, Dri M, Hall M R, et al. Waste materials for carbon capture and storage by mineralisation(CCSM)-A UK perspective[J]. App-lied Energy, 2012,99:545-554.
|
| [5] |
Bobicki E R, Liu Q, Xu Z, et al. Carbon capture and storage using alkaline industrial wastes[J]. Progress in Energy and Combustion Science, 2012,38(2):302-320.
|
| [6] |
Ji L, Yu H, Li K K, et al. Integrated absorption-mineralisation for low-energy CO2 capture and sequestration[J]. Applied Energy, 2018,225:356-366.
doi: 10.1016/j.apenergy.2018.04.108
|
| [7] |
Ji L, Yu H, Yu B, et al. Integrated absorption-mineralisation for en-ergy-efficient CO2 sequestration:Reaction mechanism and feasibili-ty of using fly ash as a feedstock[J]. Chemical Engineering Journal, 2018,352:151-162.
doi: 10.1016/j.cej.2018.07.014
|
| [8] |
Song H J, Lee S, Park K, et al. Simplified estimation of regeneration energy of 30% sodium glycinate solution for carbon dioxide absorp-tion[J]. Industrial & Engineering Chemistry Research, 2008,47:9925-9930.
|
| [9] |
Yu B, Yu H, Li K, et al. Characterisation and kinetic study of car-bon dioxide absorption by an aqueous diamine solution[J]. App-lied Energy, 2017,208(1):1308-1317.
|
| [10] |
Richner G, Puxty G. Assessing the chemical speciation during CO2 absorption by aqueous amines using in situ FTIR[J]. Industrial & Engineering Chemistry Research, 2012,51(44):14317-14324.
|
| [11] |
Robinson K, Mccluskey A, Attalla M I. An FTIR spectroscopic stu-dy on the effect of molecular structural variations on the CO2 absorp-tion characteristics of heterocyclic amines[J]. Chem.Phys.Chem., 2011,12(6):1088-1099.
doi: 10.1002/cphc.201001056
|
| [12] |
Robinson K, Mccluskey A, Attalla M I. An ATR-FTIR study on the effect of molecular structural variations on the CO2 absorption cha-racteristics of heterocyclic amines,Part Ⅱ[J]. Chem.Phys.Chem., 2012,13(9):2331-2341.
doi: 10.1002/cphc.201200066
pmid: 22517608
|
| [13] |
Max J J, Trudel M, Chapados C. Infrared titration of aqueous gly-cine[J]. Applied Spectroscopy, 1998,52(2):226-233.
doi: 10.1366/0003702981943284
|
 ),Sun Qiaoyi1,Ma Linge1,Zhuo Jinde1(
),Sun Qiaoyi1,Ma Linge1,Zhuo Jinde1( ),Song Weiguo2
),Song Weiguo2