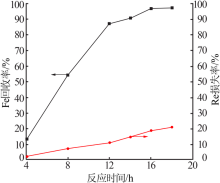
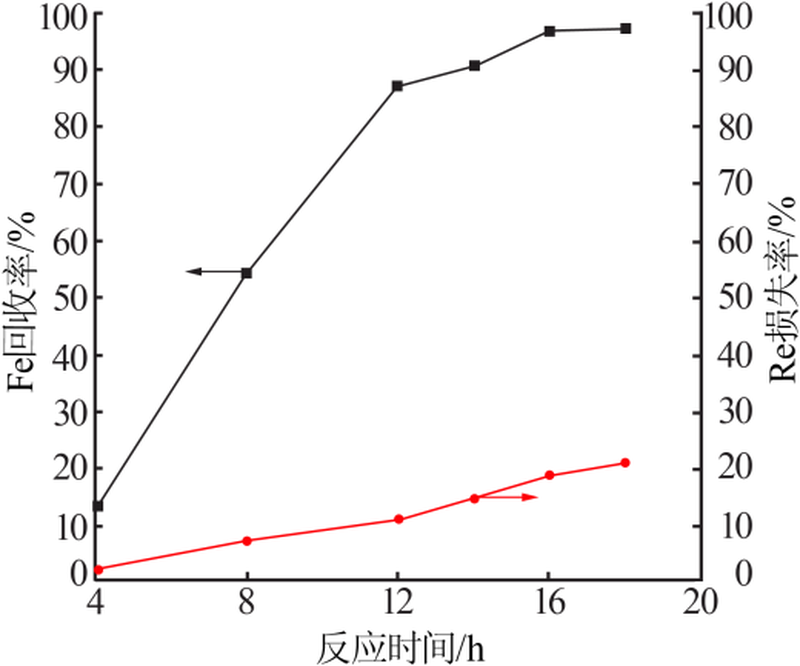
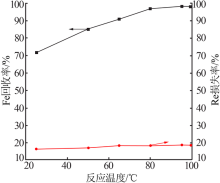
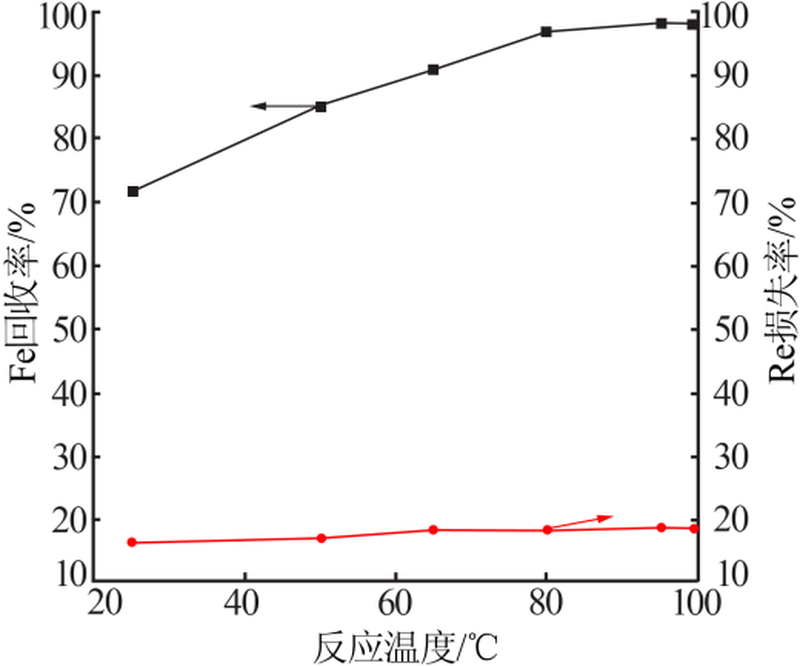
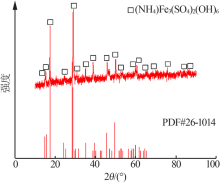
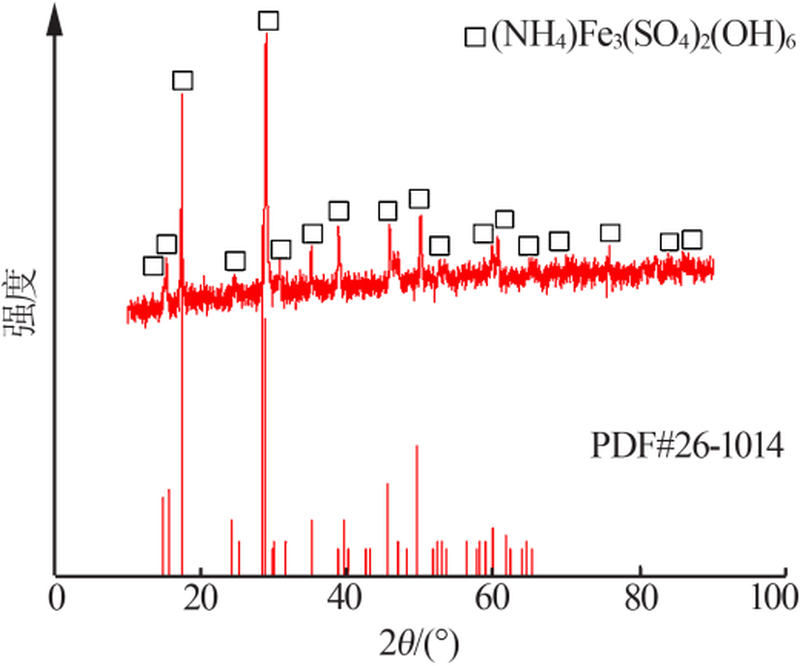
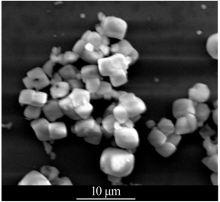
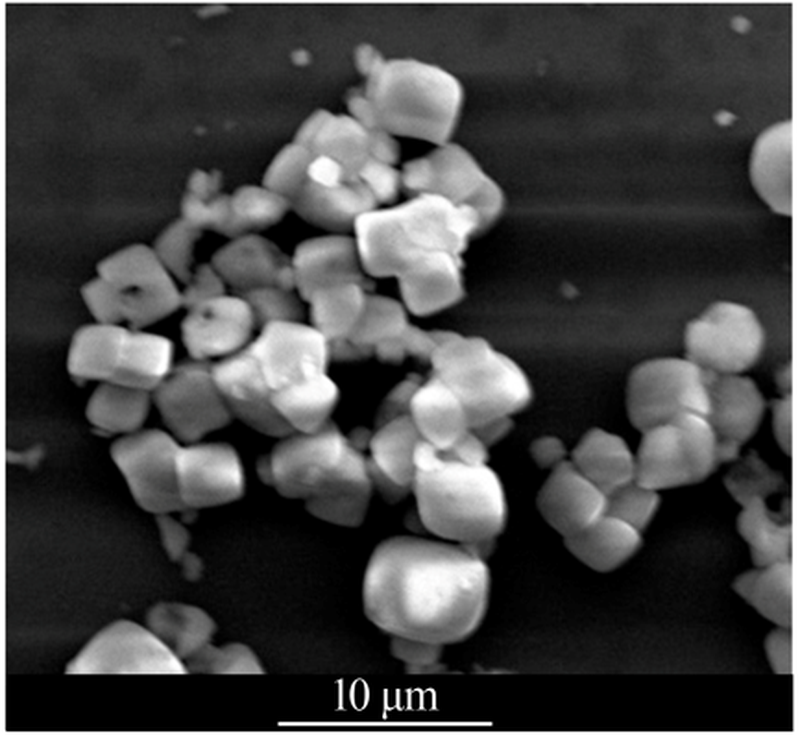
| [1] |
巫剑, 徐鹏, 吴玉春 , 等. 钕铁硼废料综合利用研究现状[J]. 山西冶金, 2018,41(1):48-50.
|
| [2] |
邓华军, 段月红, 邓庚凤 . 钕铁硼废料中稀土的回收[J]. 矿冶工程, 2019,39(1):76-78.
|
| [3] |
宋宁, 钟晓林, 龚斌 , 等. 钕铁硼二次废渣微波加热制备锰锌铁氧体[J]. 稀有金属, 2008,32(4):454-458.
|
| [4] |
王兴尧, 陈莉 .一种由钕铁硼二次废料制备纳米氧化铁红的方法:中国,107055627[P]. 2017-08-18.
|
| [5] |
Liu P F, Zhang Y F, Wang L , et al. Thermodynamics and nucleation mechanism of ammonium jarosite in sulfuric acid solution[J]. Jour-nal of Crystal Growth, 2017,478:52-57.
|
| [6] |
朱来东, 田勇, 李辉 , 等. 红土镍矿选择性溶出制备黄铵铁矾花球状晶体[J].有色金属工程, 2017(1):36-39,54.
|
| [7] |
唐明洁, 黄炳行, 明宪权 , 等. 黄铵铁矾法除铁同时浸出碳酸锰矿的研究[J].有色金属:冶炼部分, 2018(6):16-18.
|
| [8] |
徐扬, 王海峰, 任倩 , 等. 高铁菱锰矿浸出液黄铁矾法除铁实验研究[J].山西化工, 2015(3):16-18.
|
| [9] |
XB/T 617.4—2014 钕铁硼合金化学分析方法第4部分:铁量的测定——重铬酸钾滴定法[S].
|
| [10] |
XB/T 617.1—2014 钕铁硼合金化学分析方法第1部分:稀土总量的测定——草酸盐重量法[S].
|
| [11] |
常龙娇, 刘佳囡, 刘连利 , 等. 红土镍矿制备黄钠铁矾的研究[J]. 矿冶, 2018,27(3):56-59.
|
| [12] |
屈欣轲, 王雨红, 陈南雄 , 等. 黄铵铁矾法脱除高铁氧化锰矿浸出液中的铁[J]. 广西大学学报:自然科学版, 2016,41(5):1622-1628.
|
| [13] |
李晓非 . 利用硫酸铁去除废水中高浓度氨氮的研究[D]. 南京:南京大学, 2011.
|
| [14] |
Dutrizac J E . The behaviour of the rare earths during the precipita-tion of sodium,potassium and lead jarosites[J]. Hydrometallurgy, 2004,73(1/2):11-30.
|
| [15] |
刘新锋, 张丽清, 周华锋 , 等. 黄铵铁矾的制备及其催化性能[J]. 中南大学学报:自然科学版, 2011,42(12):3657-3662.
|
| [16] |
王长秋, 马生凤, 鲁安怀 , 等. 黄钾铁矾的形成条件研究及其环境意义[J]. 岩石矿物学杂志, 2005,24(6):607-611.
|
| [17] |
Basciano L C, Peterson R C . The crystal structure of ammoniojaro-site,(NH4)Fe3(SO4)2(OH)6 and the crystal chemistry of the ammo-niojarosite-hydronium jarosite solid-solution series[J]. Mineralo-gical Magazine, 2007,71(4):427-441.
|
 )
)