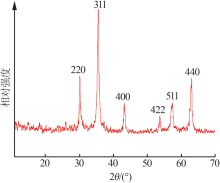
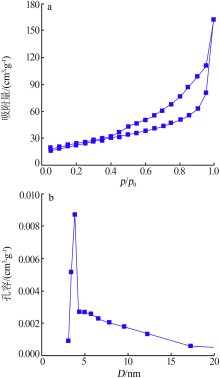
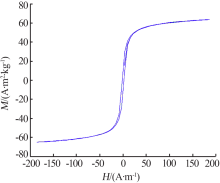
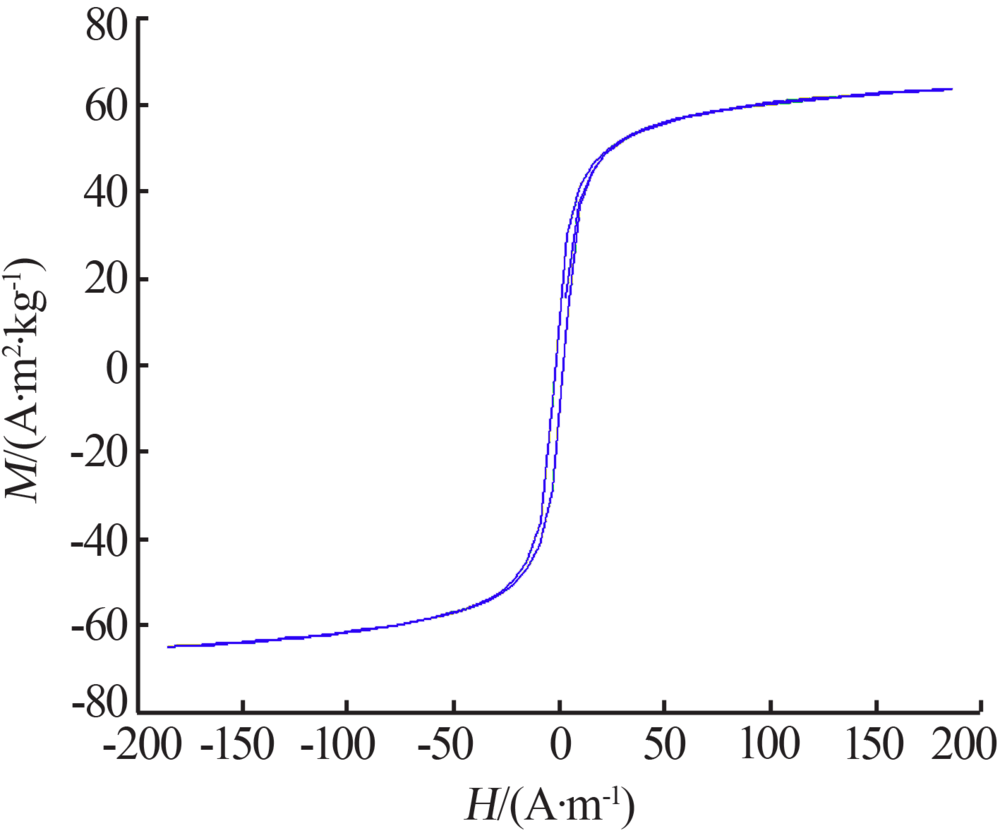
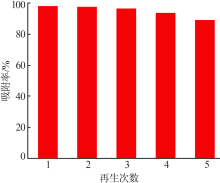
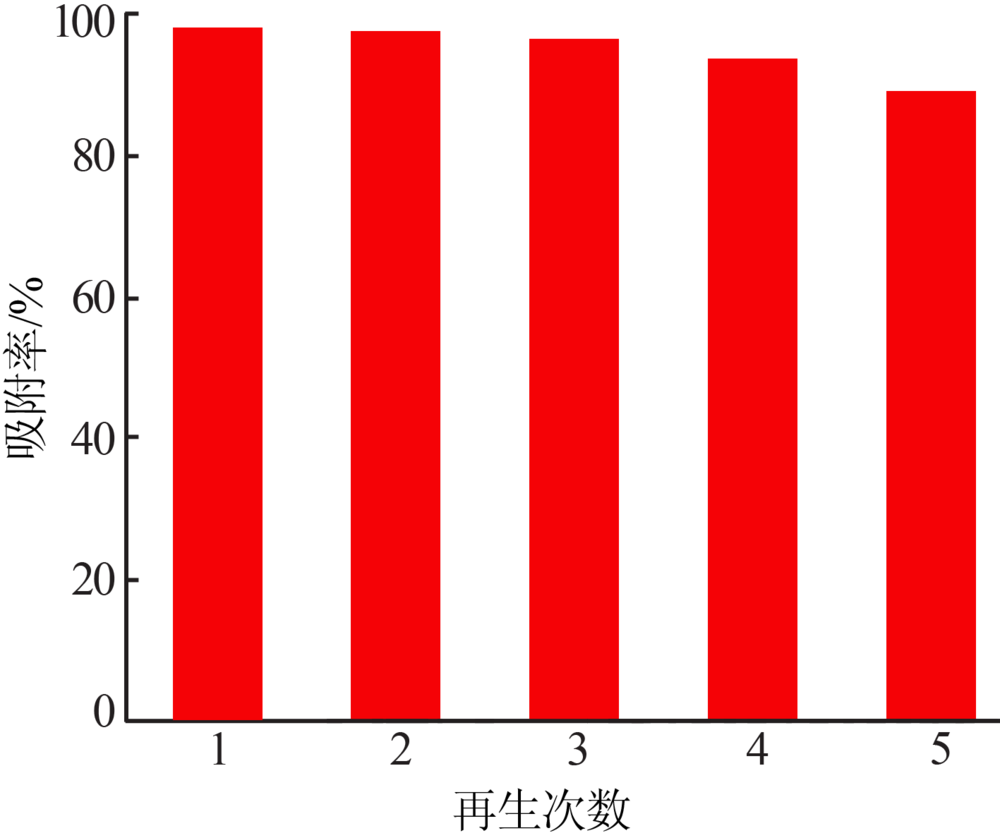
Inorganic Chemicals Industry ›› 2019, Vol. 51 ›› Issue (6): 76-79.
• Catalytic Materials • Previous Articles Next Articles
Preparation of γ-Fe2O3 nanoparticles and its adsorption properties
Zhang Bin1,Han Yuxia1,Zhao Siqin1,2, S.Asuha1,2( )
)
- 1. College of Chemical and Environmental Science,Inner Mongolia Normal University,Hohhot 010022,China
2. Inner Mongolia Key Laboratory of Functional Materials of Physics and Chemistry,Inner Mongolia Normal University
-
Received:2019-01-21Online:2019-06-10Published:2020-05-12 -
Contact:S.Asuha E-mail:asuha@imnu.edu.cn
CLC Number:
Cite this article
Zhang Bin,Han Yuxia,Zhao Siqin, S.Asuha. Preparation of γ-Fe2O3 nanoparticles and its adsorption properties[J]. Inorganic Chemicals Industry, 2019, 51(6): 76-79.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] | 滕沙沙, 李碧 . 凹凸棒石/γ-Fe2O3/碳纳米复合材料吸附有机物污染物研究[J]. 广州化工, 2013,41(11):111-113,145. |
| [2] | 米敬, 李北罡 . 不同粉煤灰对弱酸性深蓝5R的吸附性能研究[J]. 内蒙古农业大学学报:自然科学版, 2014,35(4):64-70. |
| [3] | 彭书传, 王诗生, 陈天虎 , 等. 凹凸棒石吸附水溶性染料的热力学研究[J]. 硅酸盐学报, 2005,33(8):1012-1017. |
| [4] | 张腾, 王勇梅, 彭昌盛 , 等. 染料废水的处理方法及研究进展[J]. 环保科技, 2016,22(1):36-40. |
| [5] | 霍晓源, 黄小惠 . 对染料废水处理技术的几点探讨[J]. 资源节约与环保, 2015(6):59. |
| [6] | 李乐, 成彬, 廖琪 , 等. 吸附法提取低浓度铀的研究进展[J]. 应用化工, 2017,46(3):537-541. |
| [7] | 桑亚非, 赫玉欣, 张丽 , 等. 磁性纳米材料的制备与应用进展[J]. 化工新型材料, 2017,45(1):1-3. |
| [8] | 姬丽琴, 齐永新, 周林成 , 等. 磁性微/纳米材料吸附环境污染物的研究进展[J]. 化工新型材料, 2011,39(11):32-35. |
| [9] | 潘仲彬, 刘进军 . FePt磁性纳米颗粒的化学制备方法[J]. 功能材料, 2014,45(10):10023-10029. |
| [10] |
Yu C, Dong X, Guo L , et al. Template-free preparation of mesopor-ous Fe2O3 and its application as absorbents[J]. Journal of Physical Chemistry C, 2008,112:13378-13382.
doi: 10.1021/jp8044466 |
| [11] | 都吉雅 . 纳米γ-Fe2O3的制备及其吸附性能研究[D]. 呼和浩特:内蒙古师范大学, 2015. |
| [12] | Asuha S, Gao Y W, Deligeer W , et al. Adsorptive removal of meta-hyl orange using mesoporous maghemite[J]. Journal of Porous Ma-terials, 2011,18(5):581-587. |
| [13] | Asuha S, Zhao S, Wu H Y , et al. One step synjournal of maghemite nanoparticles by direct thermal decomposition of Fe-urea complex and their properties[J]. Journal of Alloys and Compounds, 2009,472(1/2):23-25. |
| [14] | Chen F, Xiong L, Cai M , et al. Adsorption of direct fast scarlet 4BS dye from aqueous solution onto natural superfine down particle[J]. Fibers and Polymers, 2015,16(1):73-78. |
| [1] | Lu Yuyuan,Xu Song,Su Tong,Yang Zhibin,Zhang Qiqin,Zhang Yang. Study on physical and chemical characteristics and adsorption performance of converter desulfurization slag [J]. Inorganic Chemicals Industry, 2021, 53(1): 87-90. |
| [2] | Tang Na,Gong Jingkuan,Xiang Jun. Preparation and adsorption properties of aluminum-based lithium adsorbent [J]. Inorganic Chemicals Industry, 2020, 52(8): 51-56. |
| [3] | Zhao Chuang,Fan Jingxin,Guo Chunlei,Li Ben,Li Bin,Zang Jiazhong,Wang Yang,Hou Liwei,Sun Zhenhai. Study on deactivation of diesel aromatic adsorbent [J]. Inorganic Chemicals Industry, 2020, 52(8): 98-102. |
| [4] | Zhang Wending,Deng Xiaochuan,Zhu Chaoliang,Fan Faying,Zhang Yi,Qing Binju. Preparation of lithium adsorbent from PAC and its adsorption properties [J]. Inorganic Chemicals Industry, 2020, 52(2): 12-16. |
| [5] | Bian Xiaotong,Qiu Zhaofu,Yang Ji,Yan Ruiqi,Lü Shuguang. Study on fluoride removal by modified Y zeolite particles with sodium alginate-lanthanum hydroxide [J]. Inorganic Chemicals Industry, 2020, 52(12): 86-91. |
| [6] | Ye Liuying,Zeng Dewen,Chen Chi,Li Zhuye,Li Gao,Liao Yipeng,Xu Baoming. Research progress in application of absorbent of lithium extraction from brine [J]. Inorganic Chemicals Industry, 2019, 51(3): 16-19. |
| [7] | ZUO Wei-Yuan, TONG Hai-Juan, SHI Bing-Fang. Adsorption of Mn(Ⅱ) by bentonite-activate carbon compound adsorbent [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(7): 58-. |
| [8] | WANG Tao, MENG Qing-Xiang, XU Hai-Tao, XU Zhen-Liang. Preparation and characterization of lithium ion sieve nanofibrous adsorbents [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(3): 29-. |
| [9] | XU Zhan-Wu, YUE De-Yu, ZHANG Lei, CHENG Peng-Gao, GAO Tian-Cheng, TANG Na- . Research progress of preparation and formation of lithium ion sieve [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(6): 12-. |
| [10] | YAN Hui, ZHONG Hui, CHEN Nian. Preparation of new lithium adsorbents [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(2): 38-. |
| [11] | JIE Li-Xin, ZHAO Yu, CHEN Xiao-Mian, JIE Ao. Synthesis of adsorbent for extracting lithium and research on adsorption process [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(12): 28-. |
| [12] | Zhu Xinfeng;Yang Shanjiao;Jiao Guizhi. Progress in research and application of red mud in water treatment [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(2): 0-0. |
| [13] | Zhao Qiwen;Zhang Xingru;Tu Lanying;Cui Xiaoqin. Preparation of 13X zeolite granular absorbent and study on its effect of absorbing calcium ion [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(11): 0-0. |
| [14] | Wang Xiang;Zhou Qili. Research on preparation of phosphate radical absorbent with neutralization method [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(1): 0-0. |
| [15] | Xing Shujian;Zang Jiazhong;Liu Wei;Liu Guanfeng;Sun Chunhui;Yu Haibin. Progress on industrial application of molecular sieve adsorbents [J]. INORGANICCHEMICALSINDUSTRY, 2009, 0(3): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|