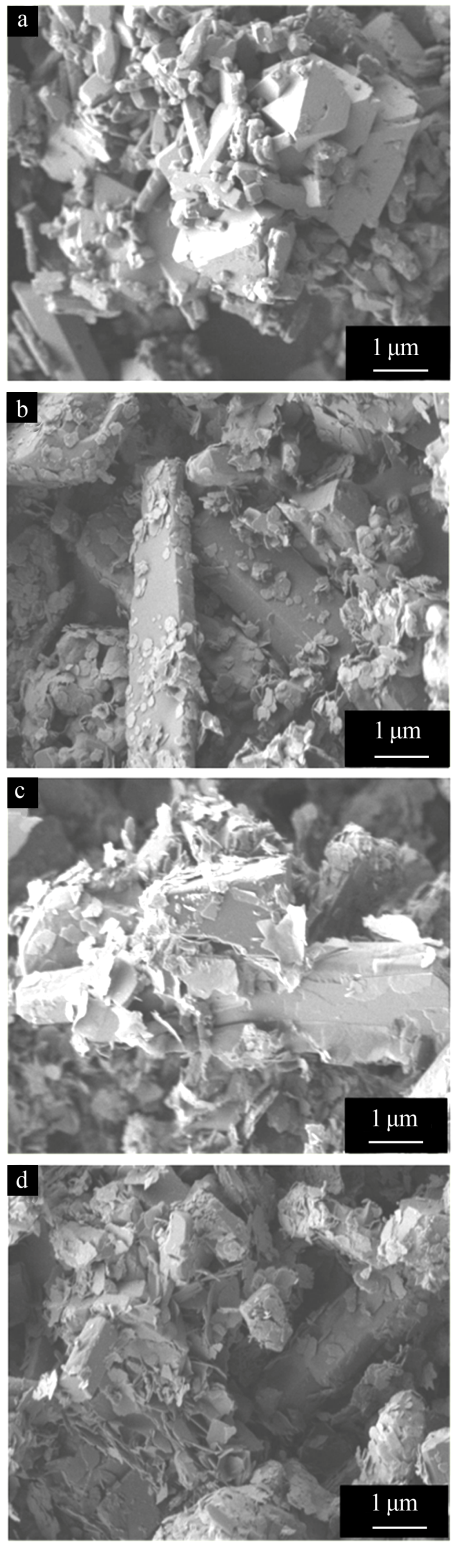
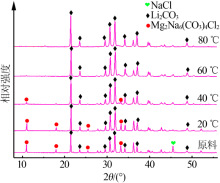
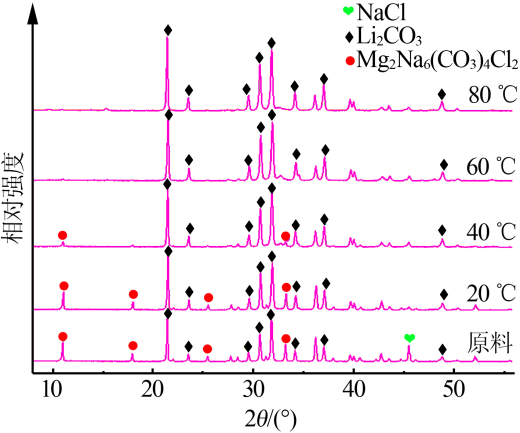
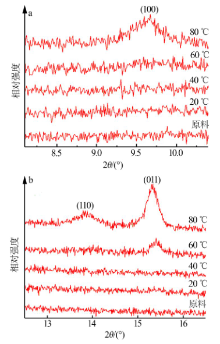
Inorganic Chemicals Industry ›› 2019, Vol. 51 ›› Issue (3): 49-52.
• Research & Development • Previous Articles Next Articles
Research on removal mechanism of soluble impurities in lithium concentrate
Hao Ziyang,Yuan Bo,Li Xin,Yi Meigui
- School of Chemical Engineering,Sichuan University,Chengdu 610065,China
-
Received:2018-09-22Online:2019-03-10Published:2020-05-09
CLC Number:
Cite this article
Hao Ziyang,Yuan Bo,Li Xin,Yi Meigui. Research on removal mechanism of soluble impurities in lithium concentrate[J]. Inorganic Chemicals Industry, 2019, 51(3): 49-52.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] |
Tarascon J M, Armand M . Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001,414:359-367.
doi: 10.1038/35104644 pmid: 11713543 |
| [2] |
邹邦坤, 丁楚雄, 陈春华 . 锂离子电池三元正极材料的研究进展[J]. 中国科学:化学, 2014,44(7):1104-1115.
doi: 10.1360/N032014-00019 |
| [3] | 孟广寿 . 矿石提锂与盐湖卤水提锂将并存发展[J].世界有色金属,2008(2):67-69. |
| [4] | 李燕茹, 朱亮, 袁建军 . 粗级碳酸锂提纯工艺过程研究[J]. 无机盐工业, 2013,45(8):15-17. |
| [5] | 邓姝皓, 杨子萱, 杨佳逸 , 等. 由扎布耶盐湖粗盐制备高纯碳酸锂新工艺研究[J]. 无机盐工业, 2016,48(4):26-30. |
| [6] | Amouzegar K, Amant G S, Harrison S .Process for the purification of lithium carbonate:US, 6048507[P]. 2000 -04-11. |
| [7] | Kang D J, Yoon M H, An J W .Method for producing high-purity li-thium carbonate:US, 9169125[P]. 2015 -10-27. |
| [8] |
Yi W, Yan C, Ma P . Crystallization kinetics of Li2CO3 from LiHCO3 solutions[J]. Journal of Crystal Growth, 2010,312(16):2345-2350.
doi: 10.1016/j.jcrysgro.2010.05.002 |
| [9] | Tsuge H . Production of lithium carbonate by gas-liquid reactive crystallization[J]. Bulletin of the Society of Sea Water Science,Japan, 2006,60(5):379-380. |
| [10] | 彭秋华, 戴扬, 徐忠吉 .深度碳化法处理碳酸盐型锂精矿生产电池级碳酸锂工艺:中国, 102502720A[P]. 2012 -06-20. |
| [11] | Hiroshi I, Hiroaki T, Katsuaki F .Production of highly pure lithium carbonate:JP, 11310414[P]. 1999 -11-09. |
| [12] | Zhang L, Liu J, Li M . Effect of different pyrogenation conditions on crystal morphology of basic magnesium carbonate[J]. Journal of the Chinese Ceramic Society, 2008,36(9):1310-1314. |
| [1] | Xu Xinfang,Zhou Xiaoping,Liu Zhengfeng,Li Lei,Chen Hu,Li Changming. Study on causticization and purification of salt lake lithium ore and recovery of fluorine-containing lithium carbonate [J]. Inorganic Chemicals Industry, 2020, 52(7): 62-65. |
| [2] | Zhang Yan,Zhao Zhenzhong,Ma Zhaohui,Zhao Yancai,Chen Zhiyu,Ma Zhengqiang. Optimization of process for preparation of battery grade lithium carbonate by carbonization [J]. Inorganic Chemicals Industry, 2020, 52(3): 68-71. |
| [3] | Hu Min,Gong Hanzhang,Wu Huadong,Guo Jia,Zhang Linfeng,Zhou Yuxin. Preparation of battery-grade lithium carbonate from lithium-containing industrial waste [J]. Inorganic Chemicals Industry, 2020, 52(3): 80-84. |
| [4] | Liu Gousheng,Wang Linlin,Liu Yuelong. Preparation of lithium carbonate from medium-and low-grade lithium clay by ammonium sulfate process [J]. Inorganic Chemicals Industry, 2020, 52(10): 125-129. |
| [5] | Chen Jie,Chen Xia. Crystallization process optimization for preparation of lithium carbonate [J]. Inorganic Chemicals Industry, 2019, 51(8): 29-32. |
| [6] | Liu Guowang,Zhou Xiaojun,Zhang Shichun,Yang Shangming,Dong Shoulong,Li Binshou. Recycle method of lithium extracted mother solution and phase diagram analysis [J]. Inorganic Chemicals Industry, 2019, 51(6): 38-40. |
| [7] | Yin Jishuai,Sun Wenliang,Hao Rusi,Wang Xiao. Study on the influence of lithium deposition reaction conditions on purity and impurities of lithium carbonate [J]. Inorganic Chemicals Industry, 2019, 51(3): 29-33. |
| [8] | Li Xin1,Yuan Bo2,Yi Meigui1. Study on removal of trace sulfur impurities from lithium carbonate by hydrothermal method [J]. Inorganic Chemicals Industry, 2019, 51(11): 28-30. |
| [9] | Hao Jiantang,Wen Fengyuan,Li Xia. Study on extracting lithium from electrolytic aluminum waste residue [J]. Inorganic Chemicals Industry, 2019, 51(10): 69-71. |
| [10] | WANG Yan-Fei, WANG Lei-Xin, XING Hong, YANG Jing, ZHAO Yan-Ping, YANG Li-Bin, ZHU Liang, ZHAO Xiao-Yu, SHA Zuo-Liang, WANG Wen-Hai. Size and morphology controlling of lithium carbonate in reactive crystallization process [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(9): 13-. |
| [11] | DENG Shu-Hao, YANG Zi-Xuan, YANG Jia-Yi, YANG Xi, YIN Chong, HAN Jiang, YI Dan-Qing. New process for preparation of high purity lithium carbonate from coarse salt in Zabuye Salt Lake [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(4): 26-. |
| [12] | TAO Zhen-Qi-1, ZHANG Zhi-Qiang-1, BI Qiu-Yan-1, NIU Hui-Zhe-1, LI Xiao-Song-2. Study on preparation of lithium carbonate via reactive crystallization from lithium chloride and sodium carbonate [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(11): 25-. |
| [13] | PENG Qiu-Hua. Production of battery-grade lithium carbonate from carbonate-type lithium concentrate by deep carbonization method [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(1): 38-. |
| [14] | LI Yan-Ru, ZHU Liang, YUAN Jian-Jun, SHA Zuo-Liang, YANG Mei-Jie, ZUO Yue-Hua. Research on purifying process of coarse lithium carbonate [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(8): 15-. |
| [15] | LI Yan-Ru, YUAN Jian-Jun, ZHU Liang, SHA Zuo-Liang, YANG Mei-Jie, ZUO Yue-Hua. Research on extraction process of lithium carbonate from salt lake brine [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(7): 12-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|