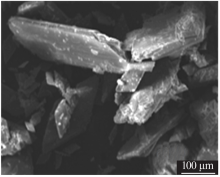
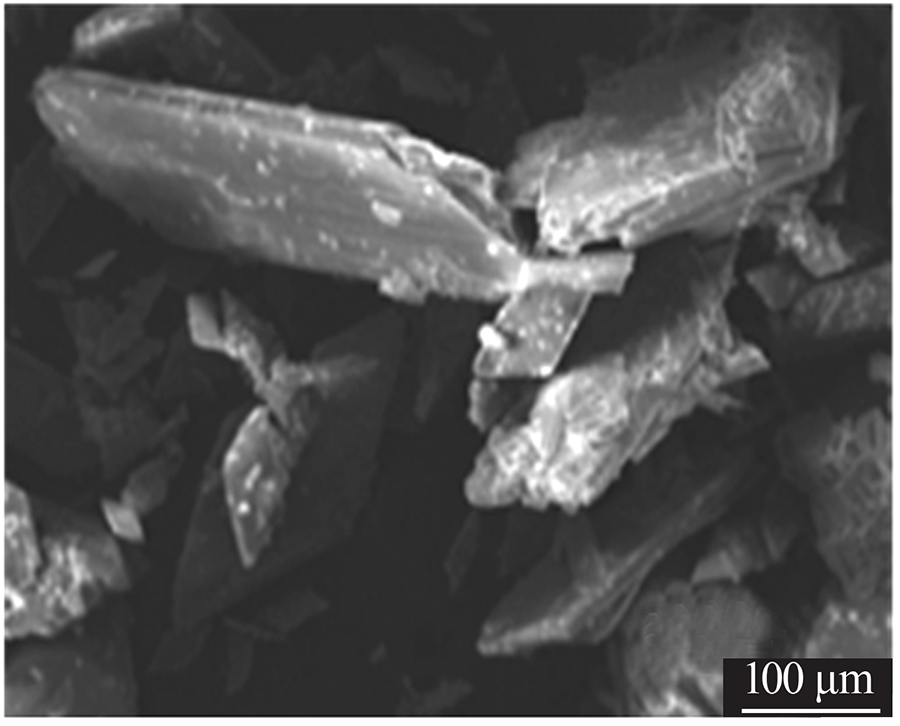
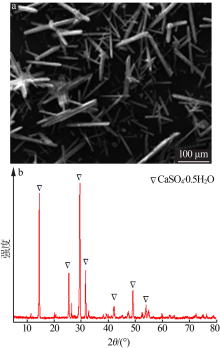
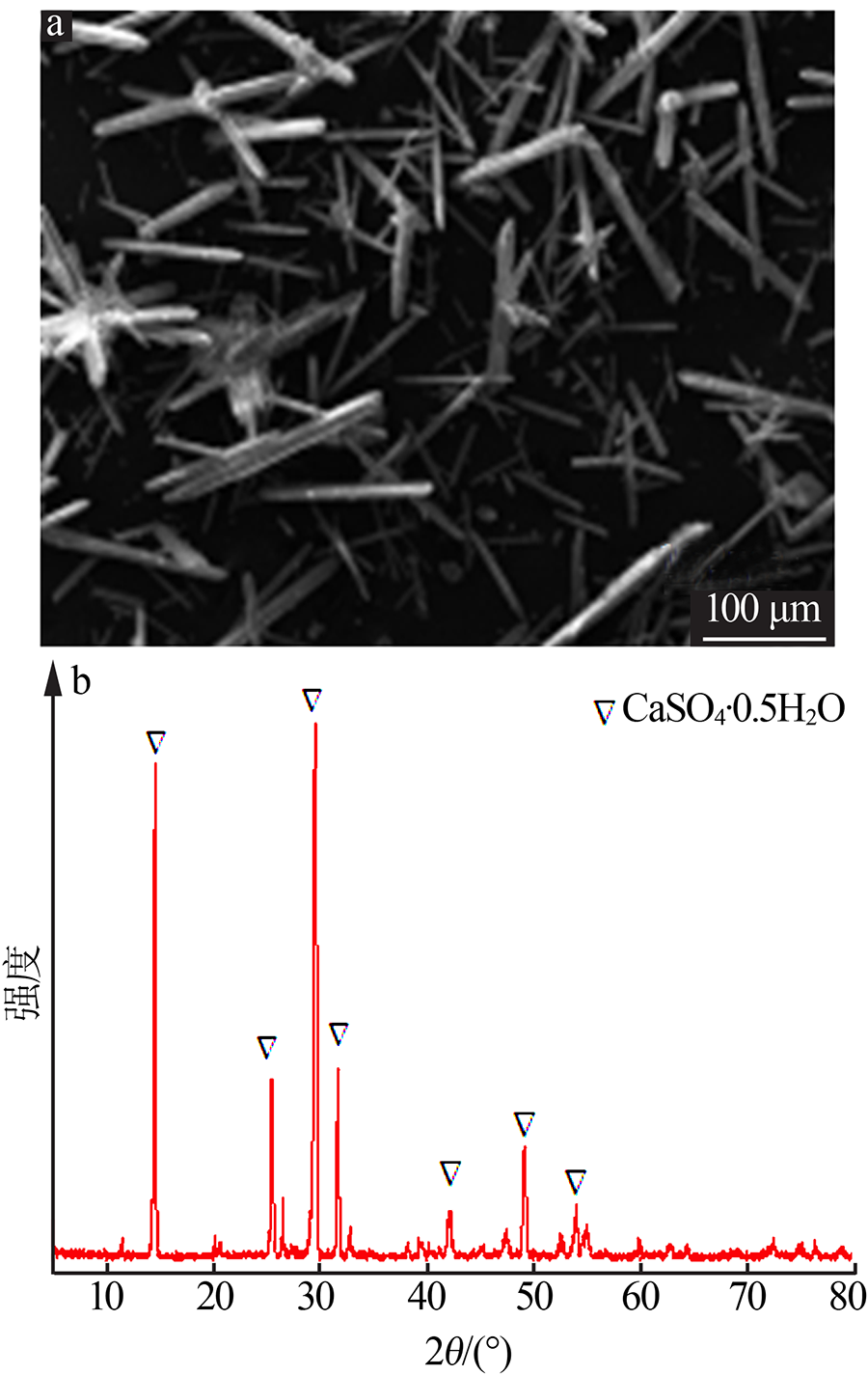
| [1] |
刘红霞 . 常压盐溶液法α-半水脱硫石膏的制备及晶形调控研究[D]. 重庆:重庆大学, 2010.
|
| [2] |
Wu X Q, Tong S T, Guan B H , et al. Transformation of flue-gas-de-sulfurization gypsum to α-hemihydrated gypsum in salt solution at atmospheric pressure[J]. Chinese Journal of Chemical Engineering, 2011,19(2):349-355.
|
| [3] |
彭家惠, 张建新, 瞿金东 , 等. 有机酸对α-半水脱硫石膏晶体生长习性的影响与调晶机理[J]. 硅酸盐学报, 2011,39(10):1711-1718.
|
| [4] |
沈卓贤 . 脱硫石膏在常压盐溶液中制备α-半水石膏的转晶剂作用研究[D]. 杭州:浙江大学, 2008.
|
| [5] |
Mi Y, Chen D, Wang S . Utilization of phosphogypsum for the preparation of α-calcium sulfate hemihydrate in chloride-free solution under atmospheric pressure[J]. Journal of Chemical Technology & Biotechnology, 2018,93(8):2371-2379.
|
| [6] |
Li F, Liu J, Yang G , et al. Effect of pH and succinic acid on the morphology of α-calcium sulfate hemihydrate synthesized by a salt solution method[J]. Journal of Crystal Growth, 2013,374:31-36.
|
| [7] |
Pan Z, Lou Y, Yang G , et al. Preparation of calcium sulfate dehydrate and calcium sulfate hemihydrate with controllable crystal morphology by using ethanol additive[J]. Ceramics International, 2013,39(5):5495-5502.
|
| [8] |
Zhao W, Gao C, Zhang G , et al. Controlling the morphology of calcium sulfate hemihydrate using aluminum chloride as a habit modifier[J]. New Journal of Chemistry, 2016,40(4):3104-3108.
|
| [9] |
Duan Z, Li J, Li T , et al. Influence of crystal modifier on the preparation of α-hemihydrate gypsum from phosphogypsum[J]. Construction & Building Materials, 2017,133:323-329.
|
| [10] |
方羊, 窦焰, 孙祥斌 , 等. Al 3+对水热法制备α-CaSO4·0.5H2O晶须的影响 [J]. 高校化学工程学报, 2017,31(2):413-419.
|
| [11] |
Zhang X, Wang J, Wu J , et al. Phase-and morphology-controlled crystallization of gypsum by using flue-gas-desulfurization gypsum solid waste[J]. Journal of Alloys and Compounds, 2016,674:200-206.
|
| [12] |
Guan B, Jiang G, Wu Z , et al. Preparation of α-calcium sulfate hemihydrate from calcium sulfate dihydrate in methanol-water solution under mild conditions[J]. Journal of the American Ceramic Society, 2011,94(10):3261-3266.
|
| [13] |
栾扬, 赵志曼, 全思臣 , 等. 基于密度泛函理论研究磷建筑石膏晶体表面吸附丁二酸转晶机理[J]. 材料导报, 2018,32(12):2118-2123.
|
| [14] |
Wu X Q, Tong S T, Guan B H , et al. Transformation of flue-gas-de-sulfurization gypsum to α-hemihydrated gypsum in salt solution at atmospheric pressure[J]. Chinese Journal of Chemical Engineering, 2011,19(2):349-355.
|
| [15] |
Tan H, Dong F . Morphological regulation of calcium sulfate hemihydrate from phosphogypsum[J]. Materialwissenschaft und Werks-tofftechnik, 2017,48(11):1191-1196.
|
| [16] |
Ma B G, Ru X H, Lu S W , et al. Preparation of α-calcium sulfate hemihydrate from phosphogypsum in CaCl2 solution under atmospheric pressure[J]. Advanced Materials Research, 2012,554/555/556(7):570-574.
|
 )
)