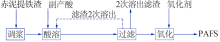
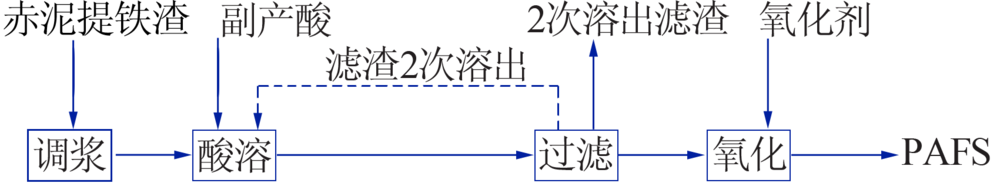
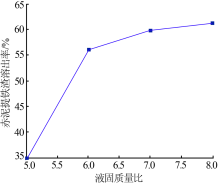
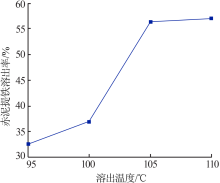
Inorganic Chemicals Industry ›› 2019, Vol. 51 ›› Issue (5): 57-60.
• Industrial Techniques • Previous Articles Next Articles
Preparation of poly ferric-aluminous-sulphate(PAFS) and its performance in phosphorous removal
Dong Linhui,Li Yang,Zhou Xuefang,Zhou Yong,Zhu Wenyuan,Zhou Xiaofeng( )
)
- Shenzhen Chang Long Technology Co.,Ltd.,Shenzhen 518116,China
-
Received:2018-11-26Online:2019-05-10Published:2020-04-28 -
Contact:Zhou Xiaofeng E-mail:zhouxiaofeng321@126.com
CLC Number:
Cite this article
Dong Linhui,Li Yang,Zhou Xuefang,Zhou Yong,Zhu Wenyuan,Zhou Xiaofeng. Preparation of poly ferric-aluminous-sulphate(PAFS) and its performance in phosphorous removal[J]. Inorganic Chemicals Industry, 2019, 51(5): 57-60.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] | 陈华, 田从学 . 国内硫酸法钛白生产现状及存在问题[J]. 攀枝花学院学报, 2014,31(1):99-102. |
| [2] | 何燕 . 国内外钛白粉生产状况与市场分析[J]. 化工时刊, 1998,12(1):37-40. |
| [3] | 熊素玉 . 硫酸法生产钛白粉的主要环境问题及对策[J]. 化工设计通讯, 2005,31(2):44-46. |
| [4] | 张华军, 李华全 . 分段阶梯法处理硫酸法钛白酸性废水的实验研究[J]. 无机盐工业, 2017,49(4):58-60. |
| [5] | 周秀梅 . 两种聚合硫酸铁生产工艺探讨[J]. 无机盐工业, 2010,42(4):39-42. |
| [6] | 韩兆元, 高玉德, 王国生 , 等. 疏水团聚-磁种法回收赤泥中铁的试验研究[J]. 金属矿山, 2011,40(3):154-156. |
| [7] | 吴菊利 . 浅析减少赤泥对环境危害的途径与方法[J]. 中国科技博览, 2014(24):141-142. |
| [8] | 刘万超, 杨家宽, 肖波 . 拜耳法赤泥中铁的提取及残渣制备建材[J]. 中国有色金属学报, 2008,18(1):187-192. |
| [9] | 徐伟勇, 杨玉峰, 王佳莹 , 等. 城市污水处理厂尾水PAC与PFS除磷对比试验研究[J]. 轻工科技, 2009,25(4):115-116. |
| [10] | 李润生, 俞明华, 聂祥 , 等. 水处理混凝剂的评价[J]. 无机盐工业, 2017,49(5):1-4. |
| [11] |
白风荣, 李赞忠, 刘进荣 . 聚合硫酸铝铁(PAFS)的合成及性能研究[J]. 工业水处理, 2014,34(12):76-77.
doi: 10.11894/1005-829x.2014.34(12).076 |
| [12] | 刘佩红, 李明玉, 李善得 , 等. 硫酸烧渣制备聚合双酸铁铝及其混凝性能[J]. 无机盐工业, 2005,37(11):49-50. |
| [13] | 刘晓, 李斌, 袁丽南 , 等. 混凝土粉末去除废水中磷的机理研究[J]. 无机盐工业, 2017,49(9):60-63. |
| [1] | Liu Junxia,Yang Yanmeng,Wang Shuaiqi,Liu Pan,Zhang Maoliang,Hai Ran. Effect of chemical activator on mechanical property of red mud geopolymer mortar [J]. Inorganic Chemicals Industry, 2020, 52(6): 72-75. |
| [2] | Hai Ran,Wang Shuaiqi,Liu Pan,Liu Junxia. Effect of thermal activated temperature on activation and mechanism of red mud from alumina production [J]. Inorganic Chemicals Industry, 2019, 51(9): 72-75. |
| [3] | HAI Ran, LIU Jun-Xia, LI Jian-Wei. Research status of radioactivity and shielding mechanism of alumina red mud [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(9): 10-. |
| [4] | GU Han-Nian, CUI Shan-Shan, WANG Ning, ZHAO Cheng-Dong. Distribution features and leaching effect of alkali in red mud [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(2): 45-. |
| [5] | JI Li-Chun, XIANG Ya-Jun. Alumina recovery from red mud by sintering method of carbide slag [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(2): 68-. |
| [6] | ZHU Xiao-Bo, LI Wang, GUAN Xue-Mao. Experiment and kinetics of dealkalization with water leaching from red mud [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(1): 41-. |
| [7] | CHEN Hong-Liang, WANG Ting, KE Yang, WANG Sheng-Bi. Technology conditions and mechanism discussion of sodium and iron extraction from red mud by acid [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(1): 44-. |
| [8] | HAN Yu-Fang, YANG Jiu-Jun, DONG Guang-Yu, MEI Shi-Gang. Research on microwave reduction Fe from Bayer red mud and thermodynamic analysis [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(2): 53-. |
| [9] | MENG Tie-Hong, LI Chun-Rong, LIU Shi-Yun. Study on preparation of PAFC from modified Bayer red mud by two stage acid leaching [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(9): 68-. |
| [10] | LIU Bang-Yu, ZHANG Wei, LIN Hua. Discussion on comprehensive utilization of red mud from bayer process in Guizhou Aluminum Works [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(10): 8-. |
| [11] | YU Yang-Hua, Wu-Yong-Gui, Yu-Li-Fei, SHEN Wan-Tun. Effect and mechanism of phosphogypsum and CaCO3 on dealkalization of red mud [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(10): 58-. |
| [12] | LI Jian-Wei, YANG Jiu-Jun, SUN Hong-Ling, ZHANG Mao-Liang, LUO Zhong-Tao. Research on technology of alkali recovery in red mud [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(1): 46-. |
| [13] | WANG Cui-Zhen, ZHOU Guang-Zhu, SONG Fang, FENG Yu. Leaching scandium from red mud by hydrochloric acid under ultrasonic oscillation [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(5): 44-. |
| [14] | LI Jian-Wei, YANG Jiu-Jun, WANG Xiao, ZHANG Mao-Liang, HAN Yu-Fang, LUO Zhong-Tao. Research on resource characteristics of red mud from sintering process [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(3): 42-. |
| [15] | ZHANG Guo-Li, LI Shao-Chun, ZHANG Xin-Yuan, WANG Zhi-Kai. Comparison study on different de-alkalization processes of red mud by Bayer process [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(8): 40-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|