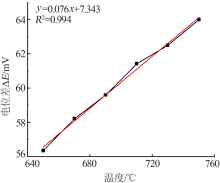
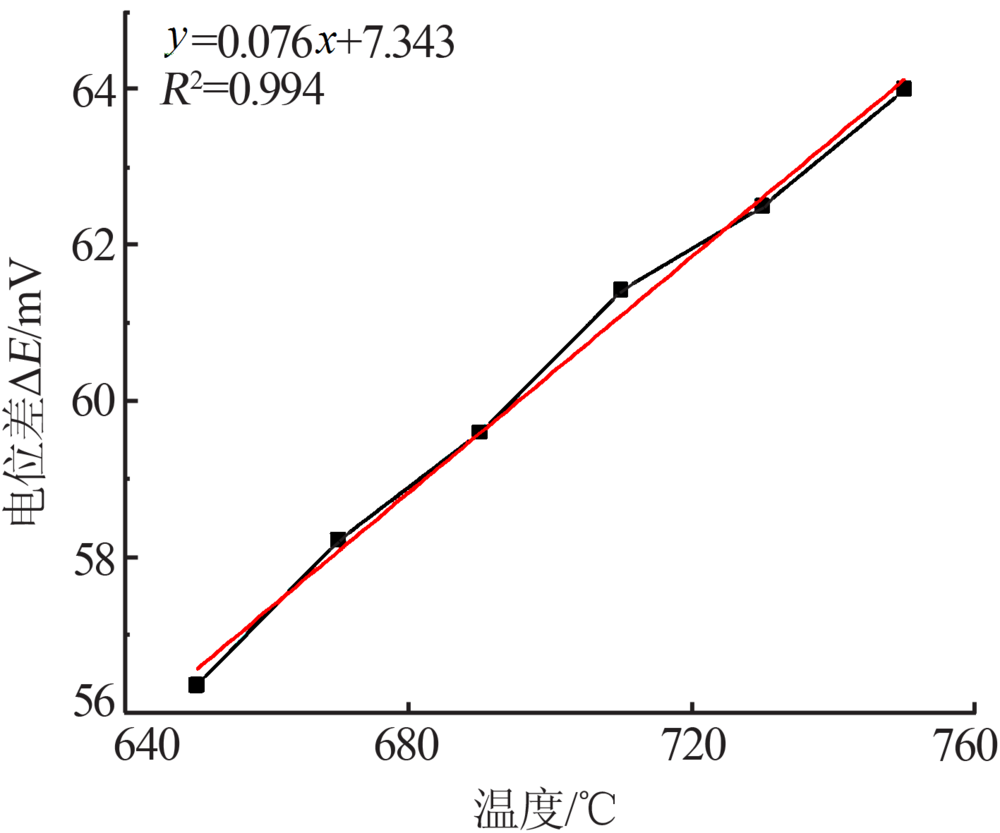
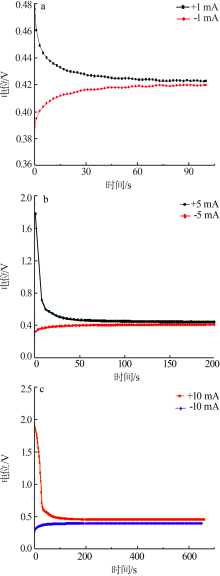
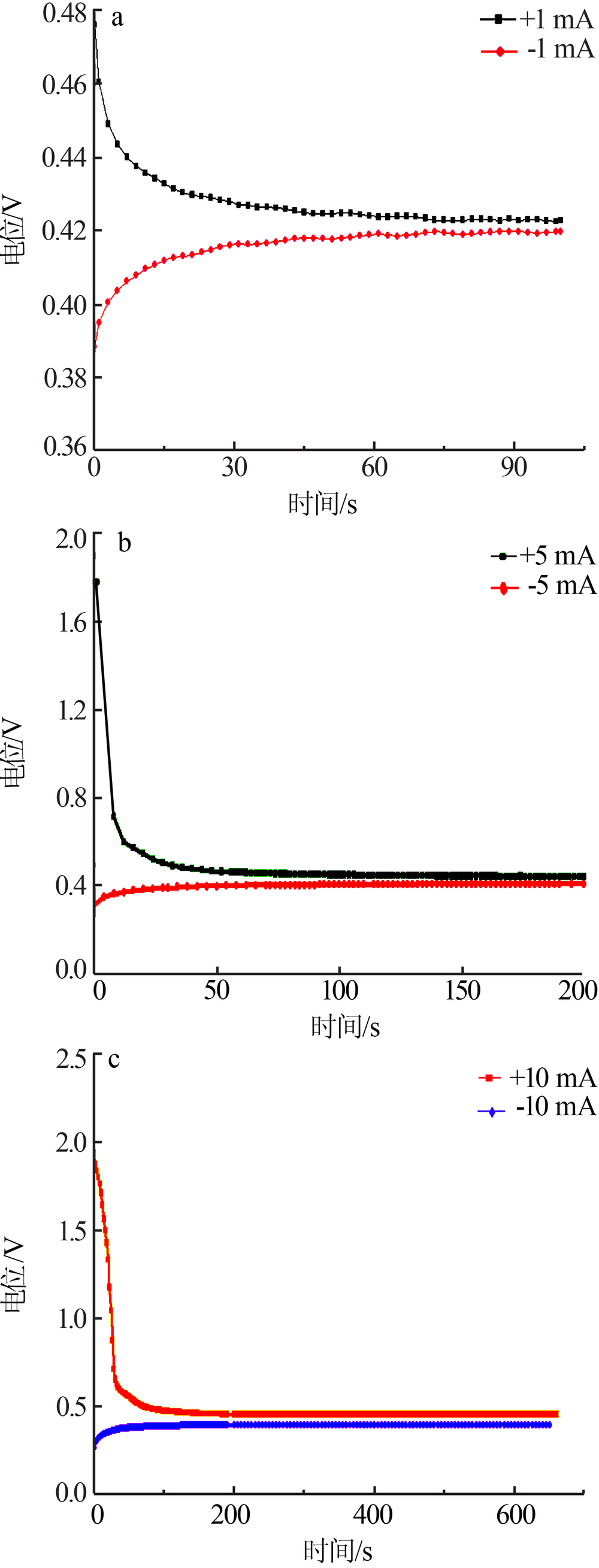
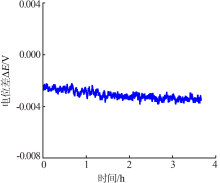
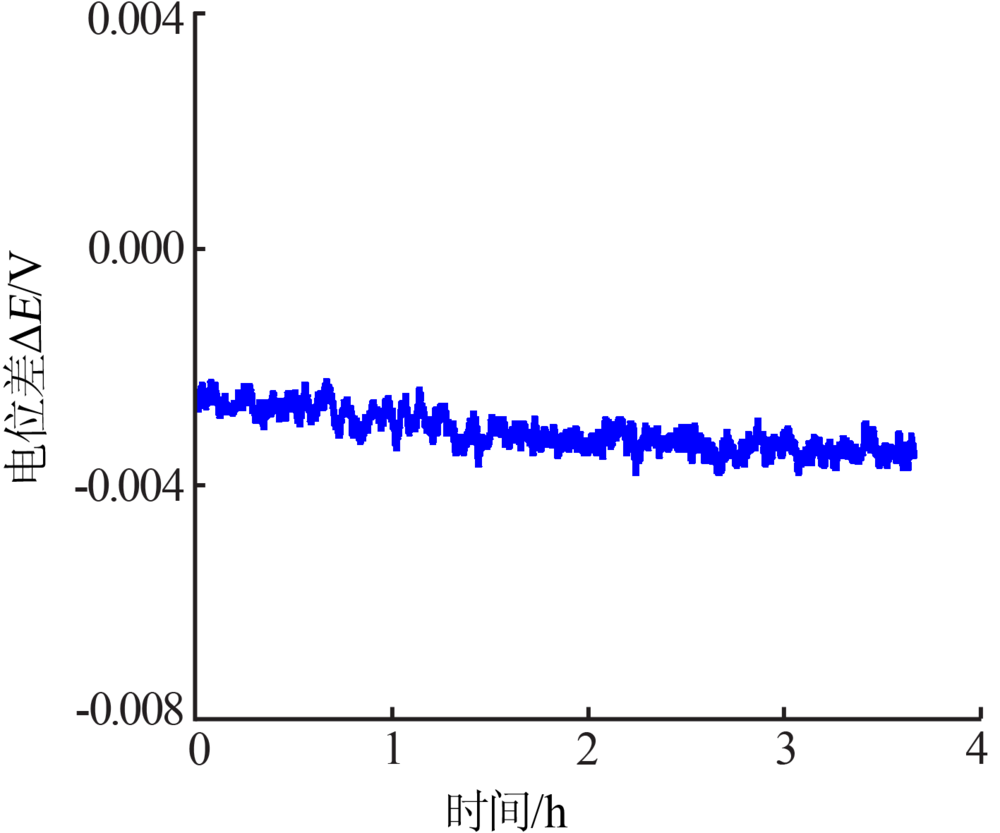
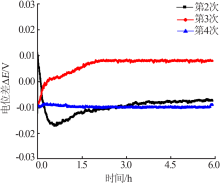
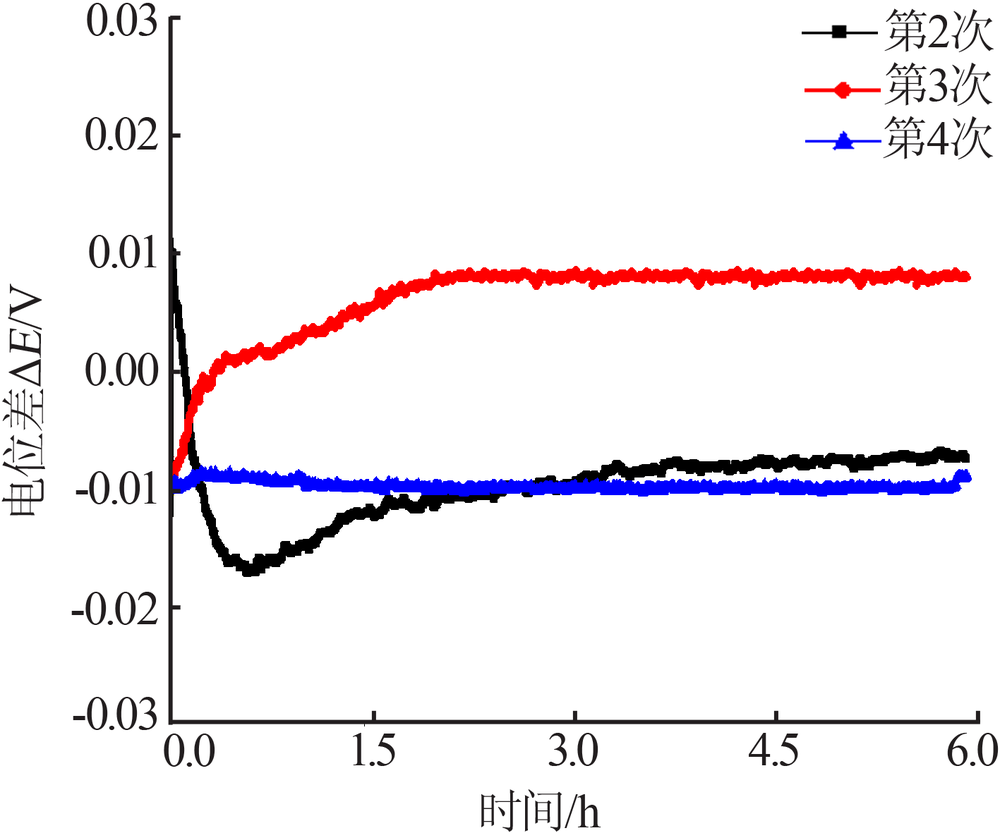
Inorganic Chemicals Industry ›› 2019, Vol. 51 ›› Issue (5): 49-52.
• Research & Development • Previous Articles Next Articles
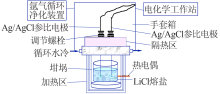
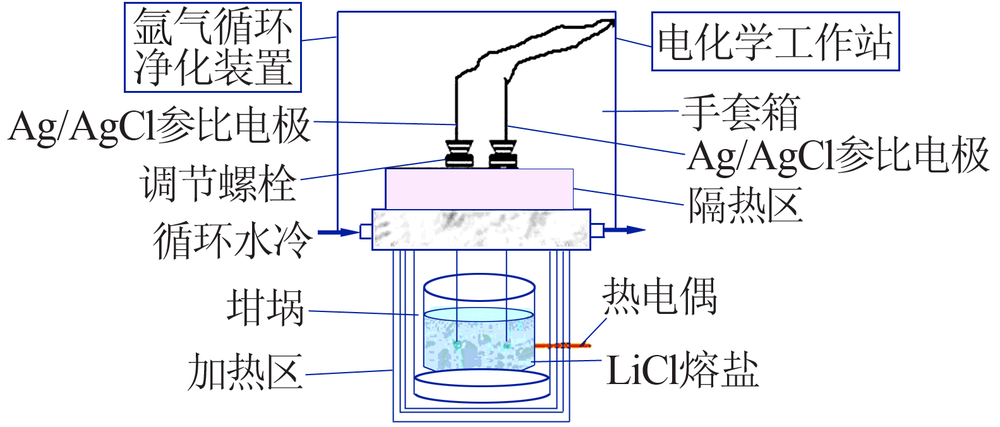
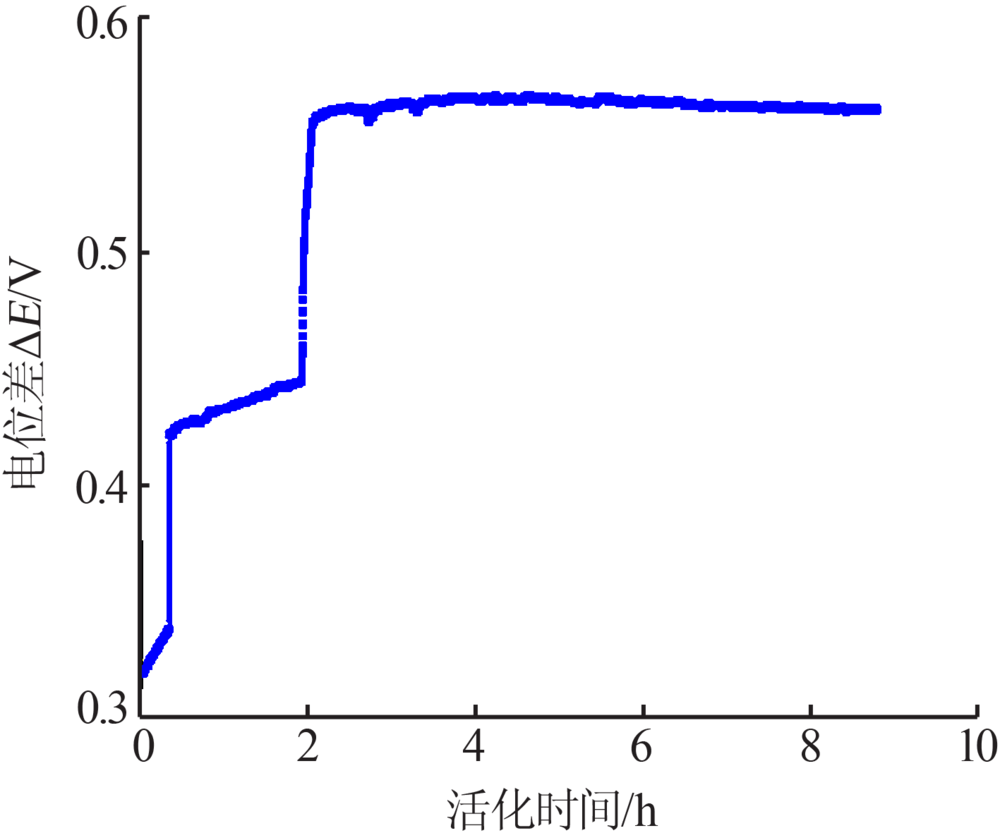
Study on performance of Ag/AgCl reference electrode in oxide electrochemical reduction system
Cheng Zhongping,He Hui,Lin Rushan,Jia Yanhong,Xiao Yiqun,Ye Guoan( )
)
- Institute of Radiochemistry,China Institute of Atomic Energy,Beijing 102413,China
-
Received:2018-11-22Online:2019-05-10Published:2020-04-28 -
Contact:Ye Guoan E-mail:yeguoan@ciae.ac.cn
CLC Number:
Cite this article
Cheng Zhongping,He Hui,Lin Rushan,Jia Yanhong,Xiao Yiqun,Ye Guoan. Study on performance of Ag/AgCl reference electrode in oxide electrochemical reduction system[J]. Inorganic Chemicals Industry, 2019, 51(5): 49-52.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] | Gourishankar K V, Redey L, Williamson M A . Electrochemical reduction of metal oxides in molten salts[R]. Illinois,US:Argonne National Lab., 2001. |
| [2] | Chen Z, She C F, Zheng H Y , et al. Electrochemical deposition of neodymium in LiF-CaF2 from Nd2O3 assisted by AlF3[J]. Electrochi-mica Acta, 2018,261:289-295. |
| [3] |
Chen Z, She C F, Zheng H Y , et al. Study on the electrochemical coreduction of Gd(Ⅲ) and Al(Ⅲ) in LiF-CaF2 melt[J]. Journal of the Electrochemical Society, 2018,165(9):D411-D416.
doi: 10.1149/2.1431809jes |
| [4] |
Alexander D T L, Schwandt C, Fray D J . The electro-deoxidation of dense titanium dioxide precursors in molten calcium chloride giving a new reaction pathway[J]. Electrochimica Acta, 2011,56(9):3286-3295.
doi: 10.1016/j.electacta.2011.01.027 |
| [5] | Xie H W, Zhao H J, Liao J Y , et al. Electrochemically controllable coating of a functional silicon film on carbon materials[J]. Electro-chimica Acta, 2018,269:610-616. |
| [6] |
Wang H, Siambun N J, Yu L P , et al. A robust alumina membrane reference electrode for high temperature molten salts[J]. Journal of the Electrochemical Society, 2012,159(9):H740-H746.
doi: 10.1149/2.033209jes |
| [7] | Kim D, Shelly X L . Simplified reference electrode for electrorefining of spent nuclear fuel in high temperature molten salt[R]. Idaho,US:Idaho National Laboratory, 2007. |
| [8] | 马樟源, 徐之强, 李裕发 , 等. 高温熔盐中使用的陶瓷隔膜银—氯化银参比电极[J]. 中国腐蚀与防护学报, 1982,2(3):47-54. |
| [9] | 谢中, 刘业翔 . 高温氯化物熔体参比电极[J]. 中国有色金属学报, 1998(4):668-672. |
| [10] | Bhatt A I, Snook G A . Reference electrodes for ionic liquids and molten salts[M] //Handbook of reference electrodes.Berlin:Springer,Springer, 2013: 189-227. |
| [11] | 贾艳虹, 何辉, 林如山 , 等. 用于熔盐高硅硼玻璃隔膜银/氯化银参比电极研究[J]. 无机盐工业, 2015,47(3):60-63. |
| [12] | Gao P, Jin X B, Wang D H , et al. A quartz sealed Ag/AgCl reference electrode for CaCl2 based molten salts[J]. Journal of Electroanaly-tical Chemistry, 2005,579(2):321-328. |
| [13] | 朱新文, 东亮, 寿洪 . 低温固相反应合成莫来石的研究[J]. 中国粉体技术, 2000,6(s1):118-121. |
| [1] | Du Zhiqiang,Yao Guangyuan. Study on characteristics of novel low-melting binary inorganic salts for heat storage [J]. Inorganic Chemicals Industry, 2020, 52(1): 63-67. |
| [2] | Shi Zhonglu,Du Peiying,Yu Xuefeng,Niu Lihui,Wang Wanqing. Study on direct preparation of potassium nitrate from nitric acid and potassium chloride [J]. Inorganic Chemicals Industry, 2019, 51(8): 37-39. |
| [3] | Zhai Wei,Yang Bo,Huang Guojia,Wang Zhigang,Li Shiping. Preparation and properties of mixed nitrite molten salts [J]. Inorganic Chemicals Industry, 2019, 51(7): 33-38. |
| [4] | WANG You-Qun, HE Hui, LIN Ru-Shan, YE Guo-An, TANG Hong-Bin, JIA Yan-Hong. Application progress of inorganic molten chlorides in dry reprocessing of spent fuel [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(8): 1-. |
| [5] | LIU Yi-Lin, GAO Yuan, BAO Jian-She, HUANG Guo-Bo, XIANG Jun-Wei. Studies on preparation and properties of functionalizing graphene doped molten salt composites [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(7): 16-. |
| [6] | JIANG Chun-Li, SONG Hong-Jun, HAN Biao, ZHAO Wei, DONG Li-Chun, HUANG Shi-You, WEN Feng-Yu. Production process of extraction chromium from chromite with sub-molten sodium hydroxide [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(7): 39-. |
| [7] | HUANG Yi-Fei, MENG Zhao-Kai, LIN Ru-Shan, JIA Yan-Hong, HE Hui, LIN Ming-Zhang. Dissolution and recovery process of CeO2 in LiCl-KCl melt [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(10): 16-. |
| [8] | JIA Yan-Hong, HE Hui, LIN Ru-Shan, TANG Hong-Bin, WANG You-Qun, CHEN Hui, YE Guo-An. Performance of boron nitride membrane Ag/AgCl reference electrode used in molten salt [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(5): 67-. |
| [9] | LIN Ru-Shan, HE Hui, YE Guo-An, TANG Hong-Bin. Decomposition voltages of CeO2 and solvents in molten salts electrolysis [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(4): 18-. |
| [10] | LUO Wei-Hua, ZHOU Zhen-Jun, LAI Zheng-Qing, LIU Chao. Synthesis of leucite from calcined bentonnite [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(9): 37-. |
| [11] | LI Hua-Quan, GUO Chuan-Hua. Comprehensive recovery of valuable elements vanadium,titanium,and tungsten from abandoned denitration catalyst [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(5): 52-. |
| [12] | WANG Jian-Hua, ZHANG Pei-Cong, QIU Yu-Chong, LI Jun-Feng. Synthesis of pure phase spinel LiMn2O4 nano-powders by molten salt method [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(4): 72-. |
| [13] | ZHAO Qian, WANG Jun-Bo, SONG Yu-Kuan, WANG Rui-Juan, AN Zhao-Peng, LIU Jiangnan. Research progress in high heat storage material of molten salt [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(11): 5-. |
| [14] | Chen Hao;Wang Xitang;Hu Qinghua;Xia Tao. Study on preparation of magnesia powder by molten salt method [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(3): 0-0. |
| [15] | Song Zuwei;Sun Huyuan;Li Xuyun;Sun Lijuan;Wang Wei;Zang Beini. Preparation of nano-]sized cobalt tungstate by low-temperature molten salt method [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(3): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|