Inorganic Chemicals Industry ›› 2019, Vol. 51 ›› Issue (5): 45-48.
• Research & Development • Previous Articles Next Articles
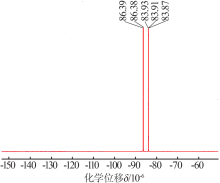
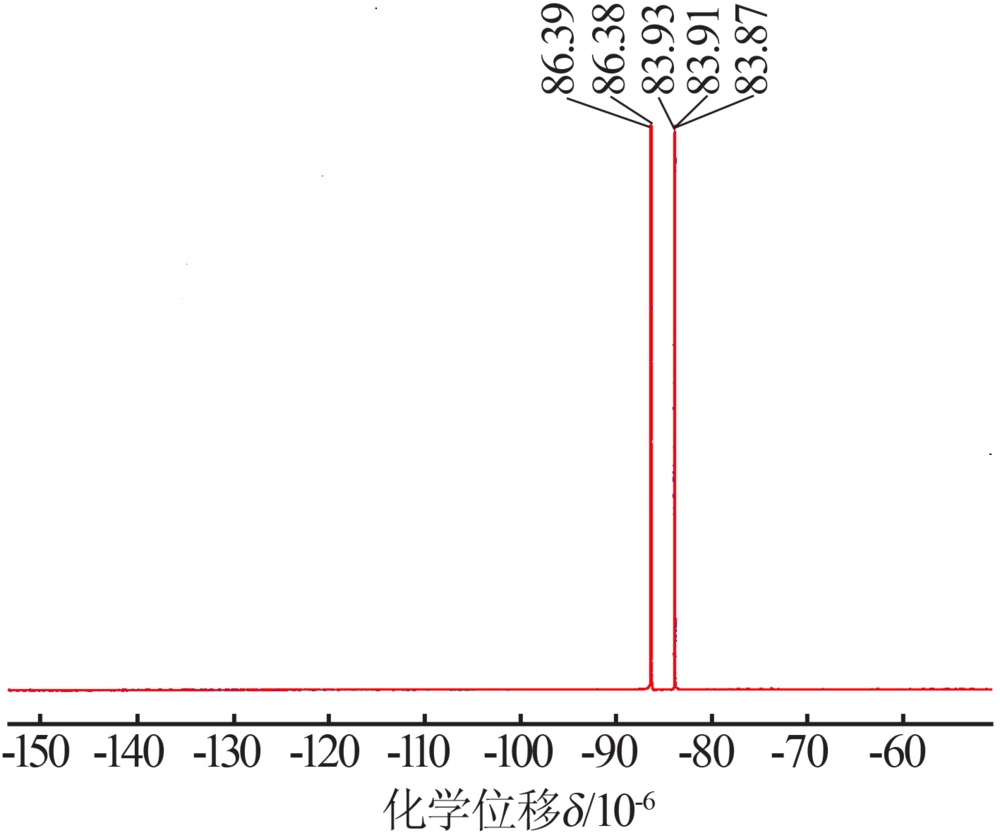
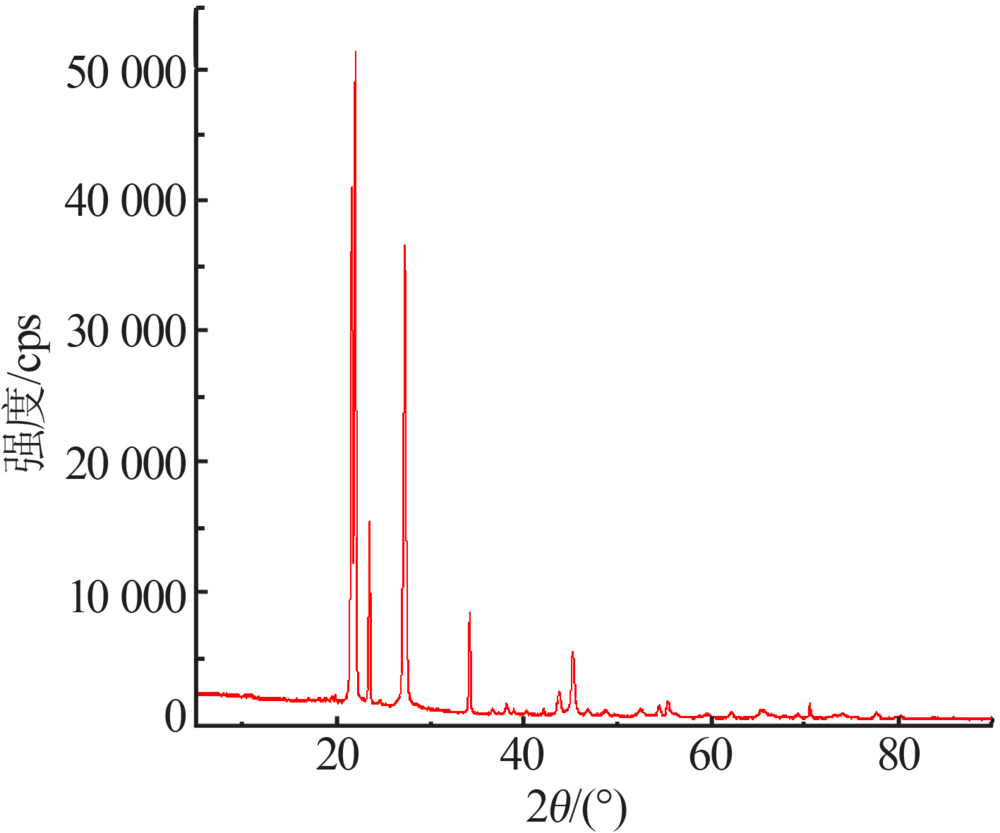
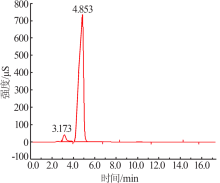
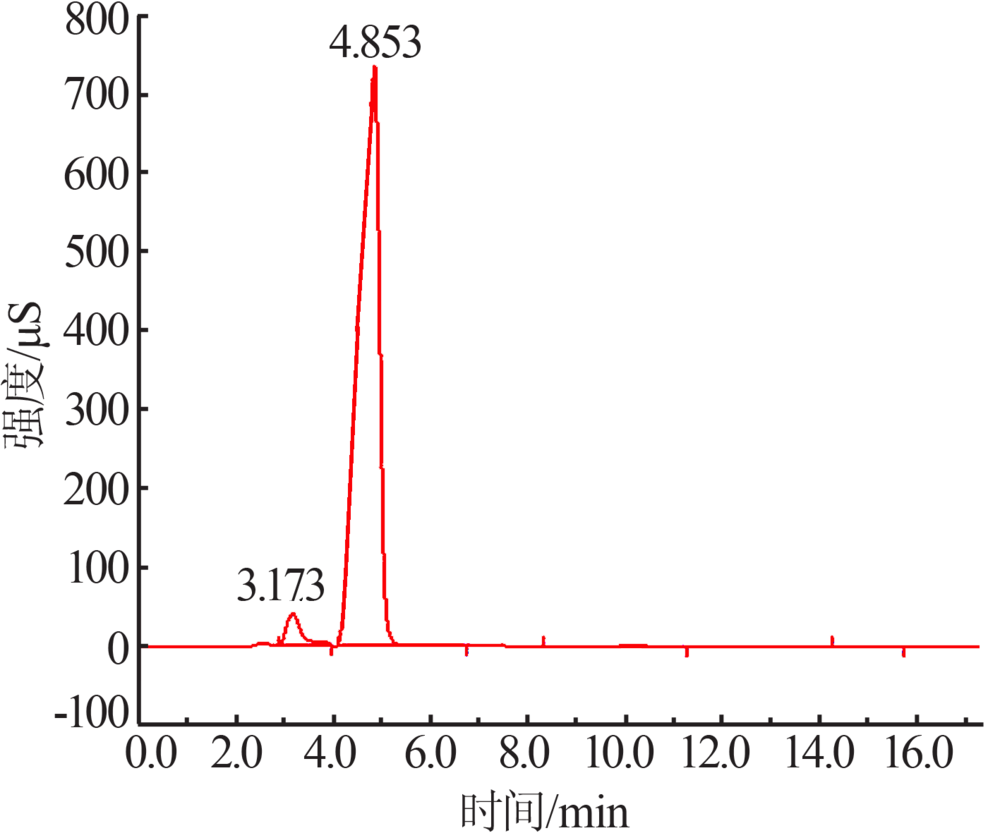
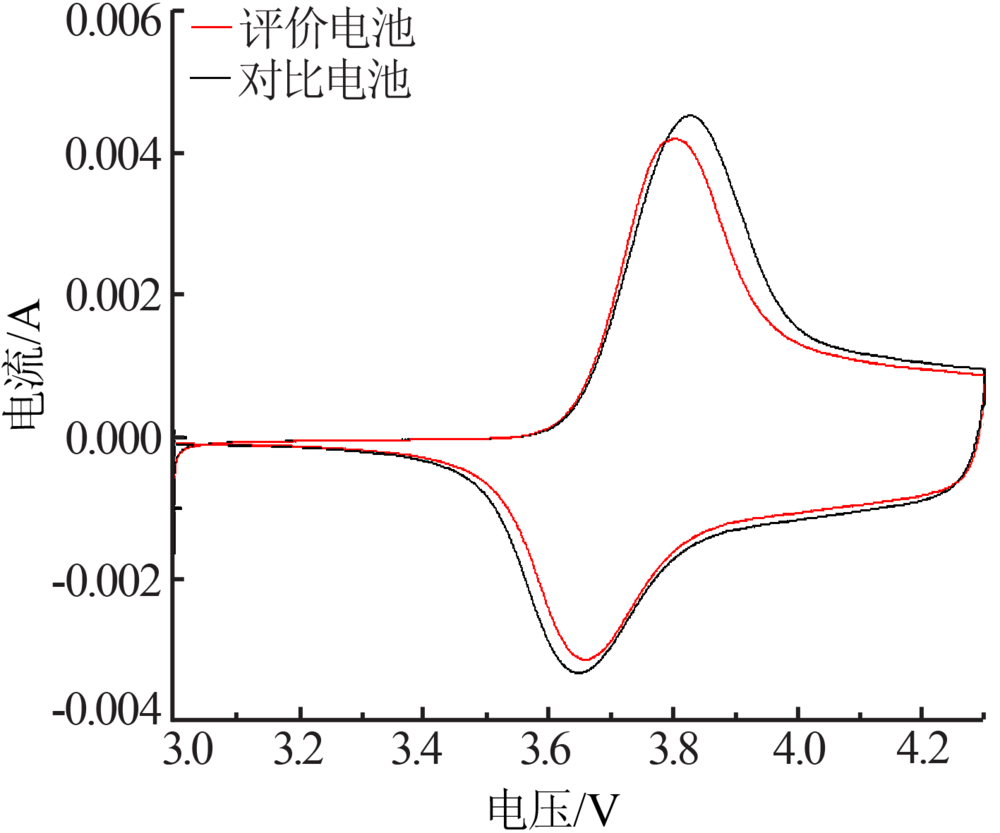
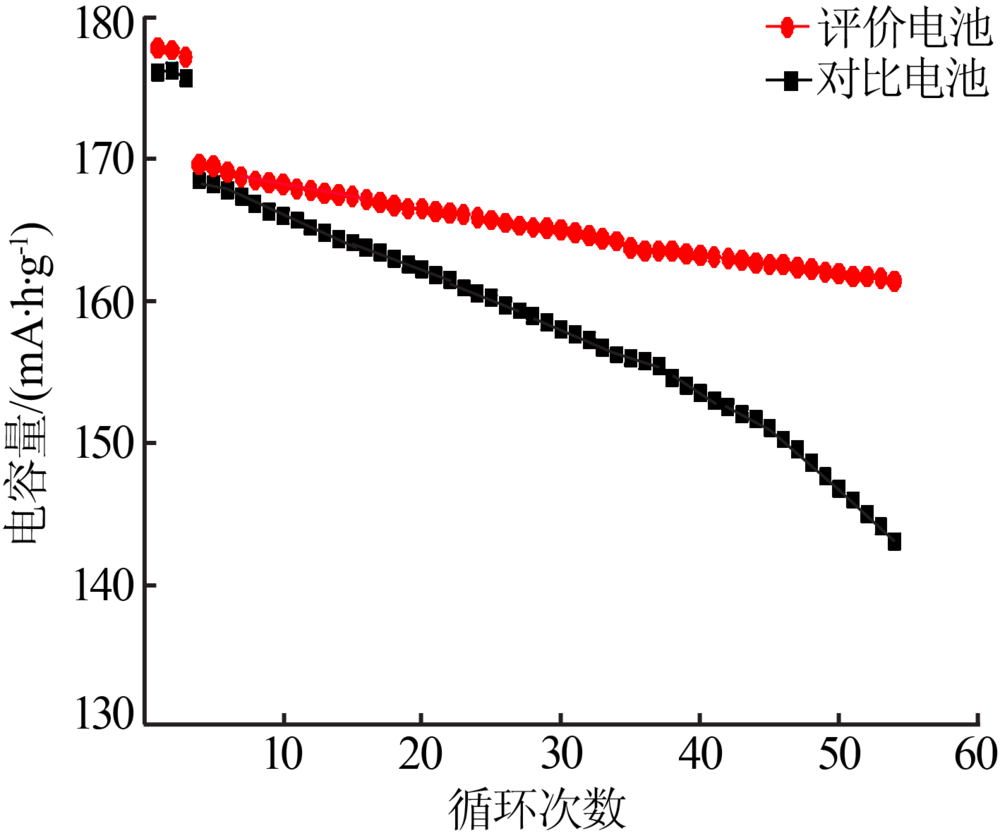
Preparation and electrochemical properties of lithium difluorophosphate
Sun Peiliang1,2,Chen Shijuan2,Yuan Li1,2
- 1. CenerTech Tianjin Chemical Research and Design Institute Co.,Ltd.,Tianjin 300131,China
2. Tianjin Jinniu Power Sources Material Co.,Ltd.