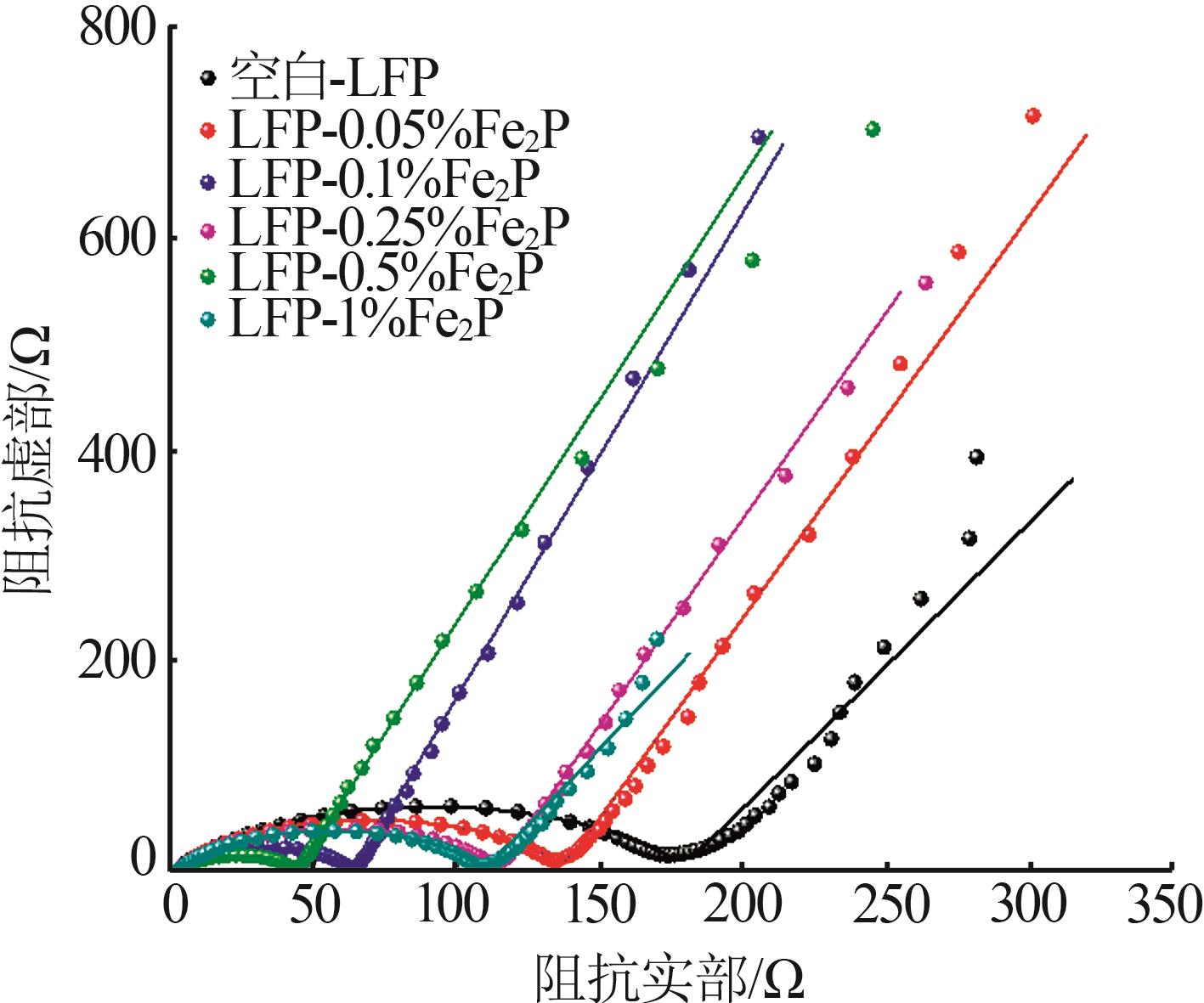
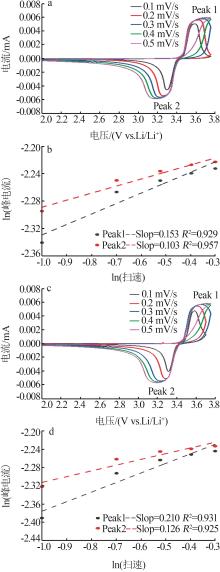
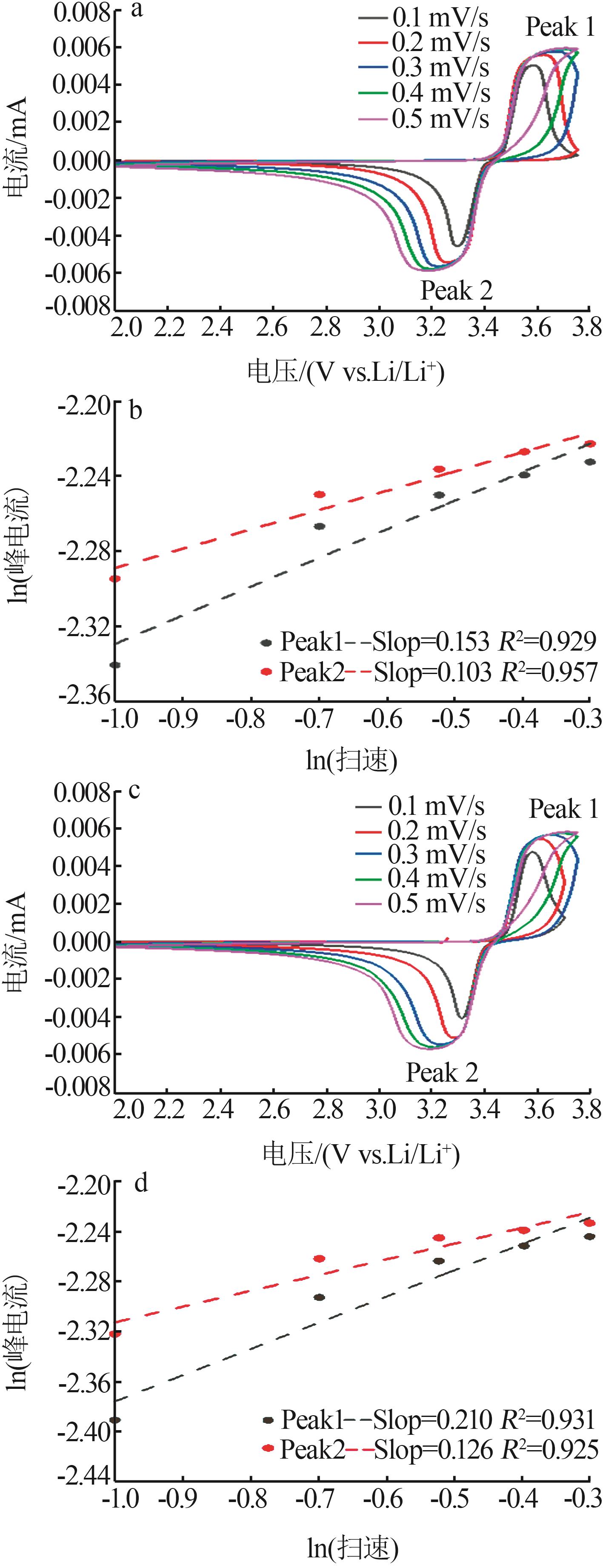
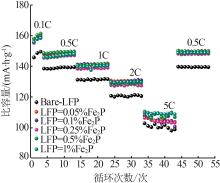
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (12): 88-94.doi: 10.19964/j.issn.1006-4990.2023-0349
• Research & Development • Previous Articles Next Articles
Study on effect of Fe2P on electrochemical performance of LiFePO4
QU Lian1,2( ), LI Yuezhu2, LI Mingya2, WANG Zhaopei1,2, CHEN Yanyu2, LI Yineng1,2(
), LI Yuezhu2, LI Mingya2, WANG Zhaopei1,2, CHEN Yanyu2, LI Yineng1,2( )
)
- 1. Qujing Dynanonic Co.,Ltd.,Qujing 655000,China
2. Foshan Dynanonic Co.,Ltd.,Foshan 528500,China
-
Received:2023-07-05Online:2023-12-10Published:2023-12-14 -
Contact:LI Yineng E-mail:qulian@dynanonic.com;liyineng@dynanonic.com
CLC Number:
Cite this article
QU Lian, LI Yuezhu, LI Mingya, WANG Zhaopei, CHEN Yanyu, LI Yineng. Study on effect of Fe2P on electrochemical performance of LiFePO4[J]. Inorganic Chemicals Industry, 2023, 55(12): 88-94.
share this article
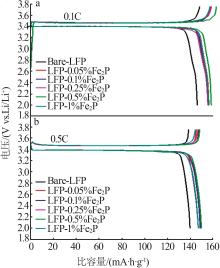
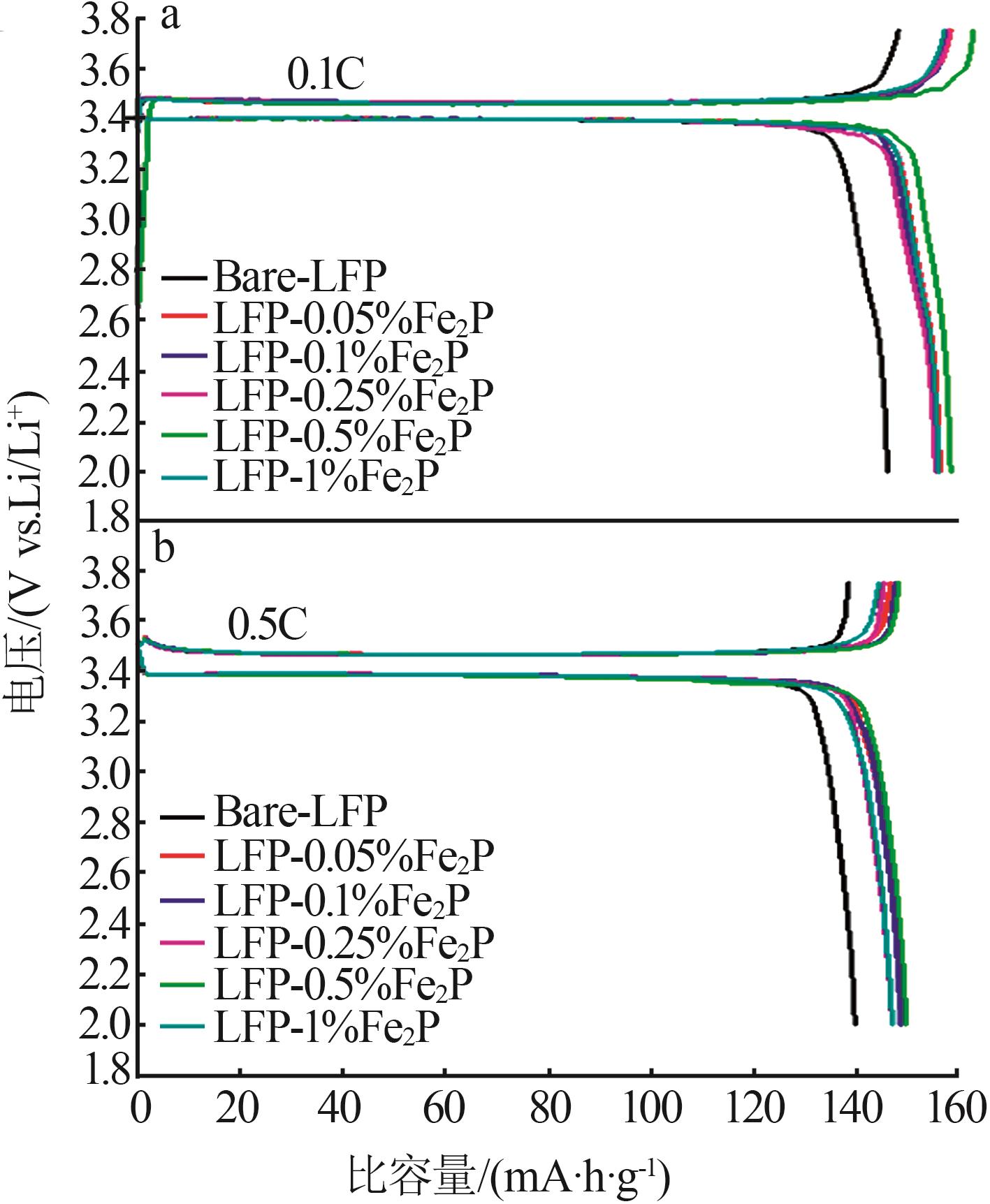
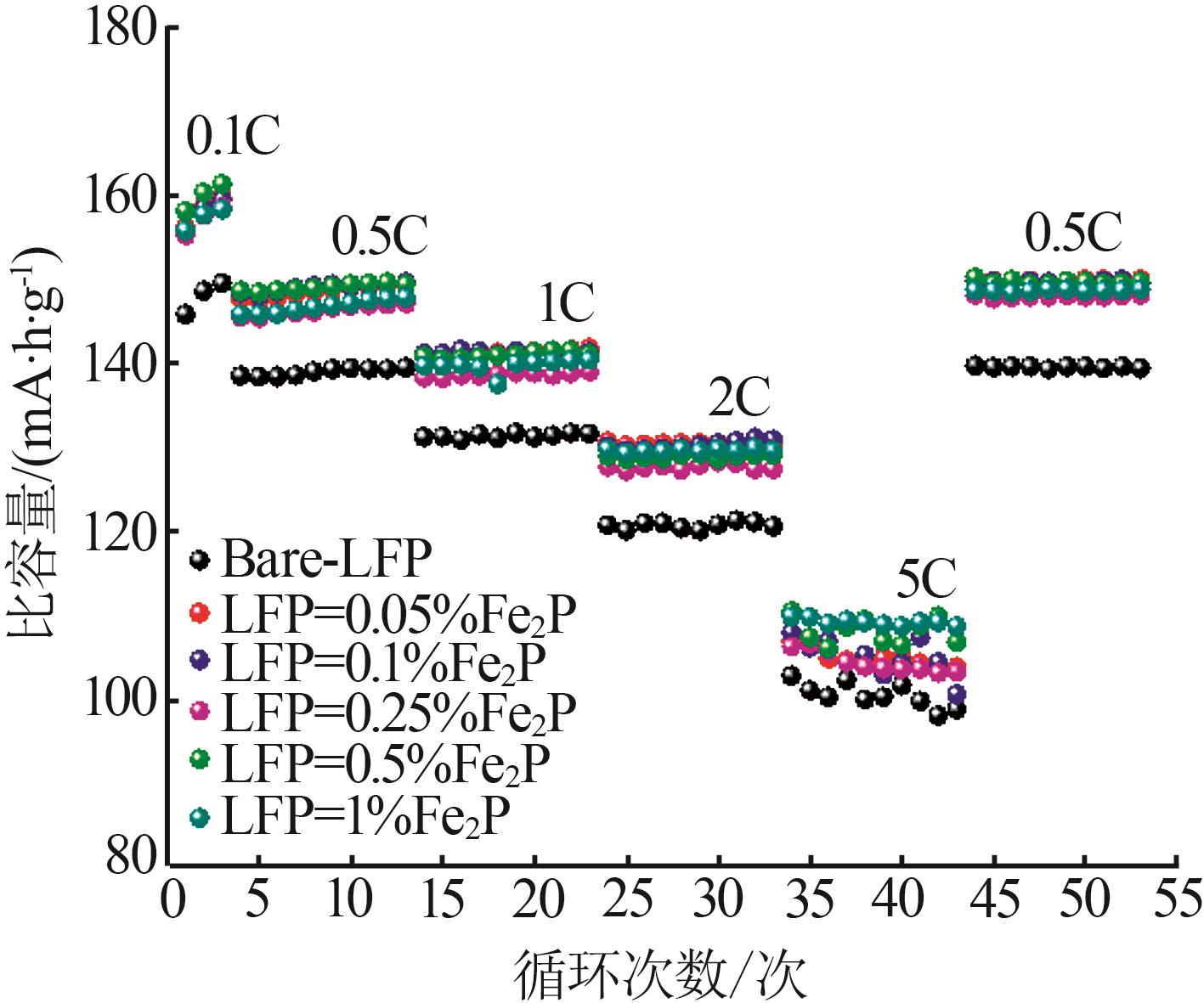
Table 1
Electrochemical performance of composite LFP samples doped with different mass fractions of Fe2Pat 0.1C and 0.5C magnification"
| 样品 | 0.1C放电 比容量/ (mA·h·g-1) | 0.1C首次 库伦效率/ % | 0.5C放电 比容量/(mA·h·g-1) | 0.5C首次 库伦效率/ % |
|---|---|---|---|---|
| Bare-LFP | 145.9 | 97.6 | 138.5 | 99.8 |
| LFP-0.05%Fe2P | 156.2 | 95.9 | 147.4 | 99.6 |
| LFP-0.1%Fe2P | 155.9 | 98.1 | 148.1 | 99.7 |
| LFP-0.25%Fe2P | 155.3 | 97.4 | 145.5 | 99.7 |
| LFP-0.5%Fe2P | 158.2 | 96.6 | 148.5 | 99.6 |
| LFP-1%Fe2P | 155.9 | 98.4 | 145.9 | 99.8 |
| 1 | 赵元元, 陈海峰, 刘云云, 等. 锰系锂离子筛的制备与改性的研究进展[J]. 无机盐工业, 2022, 54(2):21-29. |
| ZHAO Yuanyuan, CHEN Haifeng, LIU Yunyun, et al. Research progress on preparation and modification of manganese based lithium ion sieve[J]. Inorganic Chemicals Industry, 2022, 54(2):21-29. | |
| 2 | 潘晓晓, 庄树新, 孙雨晴, 等. 动力型磷酸铁锂正极材料改性的研究进展[J]. 无机盐工业, 2023, 55(6):18-26. |
| PAN Xiaoxiao, ZHUANG Shuxin, SUN Yuqing, et al. Research progress of modified-LiFePO4 as cathode materials for lithium ion batteries[J]. Inorganic Chemicals Industry, 2023, 55(6):18-26. | |
| 3 |
GUO Bangjun, LIU Chenghao, GAO Yizhao, et al. A combining electrochemical model for LiFePO4-graphite lithium-ion battery considering cathode heterogeneous solid phase phenomenon[J]. International Journal of Energy Research, 2022, 46(11):15231-15243.
doi: 10.1002/er.v46.11 |
| 4 |
KULKA A, REDEL K, MOLENDA J. Platelet-shape LiFePO4/Fe2P/C composite material as a high-rate positive electrode for Li-ion batteries[J]. Solid State Ionics, 2019, 335:113-120.
doi: 10.1016/j.ssi.2019.03.004 |
| 5 | 王强, 曾晖, 王康平. 磷酸铁锂烧结工艺对磷化铁生成的影响[J]. 电池, 2015, 45(4):209-211. |
| WANG Qiang, ZENG Hui, WANG Kangping. Effects of lithium iron phosphate calcination condition to formation of iron phosphorus[J]. Battery Bimonthly, 2015, 45(4):209-211. | |
| 6 |
RAHMAN M M, WANG Jiazhao, ZENG Rong, et al. LiFePO4-Fe2P-C composite cathode:An environmentally friendly promising electrode material for lithium-ion battery[J]. Journal of Power Sources, 2012, 206:259-266.
doi: 10.1016/j.jpowsour.2012.01.119 |
| 7 | KONAROVA M, TANIGUCHI I. Physical and electrochemical properties of LiFePO4 nanoparticles synthesized by a combination of spray pyrolysis with wet ball-milling[J]. Journal of Power Sour- ces, 2009, 194(2):1029-1035. |
| 8 | LI Zhaojin, YANG Jinxing, GUANG Tianjia, et al. Controlled hydrothermal/solvothermal synthesis of high-performance LiFePO4 for Li-ion batteries[J]. Small Methods, 2021, 5(6): e2100193. |
| 9 |
WANG Juan, ZHANG Shuang, SHAO Zhongbao, et al. Performance of Fe2P/LiFePO4/C composites synthesized by a novel and facile method[J]. Russian Journal of Physical Chemistry A, 2020, 94(13):2710-2714.
doi: 10.1134/S0036024420130300 |
| 10 |
LIU Zhihua, ZHANG Ronglan, XU Feifei, et al. Structure and electrochemical performance of LiFePO4 cathode materials modified with carbon coating and metal doping[J]. Journal of Solid State Electrochemistry, 2022, 26(8):1655-1665.
doi: 10.1007/s10008-022-05198-8 |
| 11 |
LIU Yang, QI Cai, CAI Dandan, et al. TaC-modified LiFePO4/C composite as cathode material for high-performance lithium-ion batteries[J]. Ionics, 2023, 29(6):2191-2198.
doi: 10.1007/s11581-023-04969-1 |
| 12 |
SUN Ruimin, LIU Sijie, WEI Qiulong, et al. Mesoporous NiS2 nanospheres anode with pseudocapacitance for high-rate and long-life sodium-ion battery[J]. Small, 2017, 13(39):1701744.
doi: 10.1002/smll.v13.39 |
| 13 |
LI Yin, WANG Li, ZHANG Keyu, et al. High performance of LiFePO4 with nitrogen and phosphorus dual-doped carbon layer for lithium-ion batteries[J]. Journal of Alloys and Compounds, 2022, 890:161617.
doi: 10.1016/j.jallcom.2021.161617 |
| 14 |
PENG Jiawu, HONG Xiaoting, ZHOU Qiongxiang, et al. Novel synthesis of 3D mesoporous FePO4 from electroflocculation of iron filings as a precursor of high-performance LiFePO4/C cathode for lithium-ion batteries[J]. ACS Omega, 2023, 8(14):12707-12715.
doi: 10.1021/acsomega.2c07838 pmid: 37065085 |
| [1] | CHEN Tiandong, ZHAO Guangzhao, HAI Chunxi, DONG Shengde, HE Xin, XU Qi, FENG Hang, YUAN Shaoxiong, MA Luxiang, ZHOU Yuan. Research and industrialization progress on coating and doping modification of lithium-rich manganese-based materials [J]. Inorganic Chemicals Industry, 2023, 55(9): 1-8. |
| [2] | XU Ruilin, ZENG Tao, LIU Huan, LIU Xingwei, WANG Hao, XU Xiaoming, ZHAO Lipeng. Cause analysis of early cycling attenuation of LiFePO4 battery and its performance improvement [J]. Inorganic Chemicals Industry, 2023, 55(3): 92-97. |
| [3] | XU Ruilin,ZHAO Lipeng,LIU Xingwei,LIU Huan,WANG Hao,XU Xiaoming,ZENG Tao. Improvement of high temperature cycling performance of LiFePO4 [J]. Inorganic Chemicals Industry, 2022, 54(9): 108-112. |
| [4] | HOU Shunli,ZHAO Duan,ZHOU Geng,WEI Shishi,LI Jian,WANG Jiatai. Research progress on doping modification of high nickel ternary nickel-cobalt-aluminum cathode material [J]. Inorganic Chemicals Industry, 2022, 54(8): 40-46. |
| [5] | ZHANG Xinyi,DI Yuli,DONG Qi,CHEN Xingyu,ZHANG Zhengdong. Research progress on preparation of Li3V2(PO4)3 cathode material for lithium-ion batteries [J]. Inorganic Chemicals Industry, 2022, 54(3): 38-44. |
| [6] | LU Zheng,CHEN Kunfeng,XUE Dongfeng. Study on large-scale preparation and electrochemical properties of high thermal stabilized α-Fe2O3 [J]. Inorganic Chemicals Industry, 2022, 54(3): 45-50. |
| [7] | MA Caifu,YUAN Chuanlai,ZHAO Xueqi. Effect of ball milling time on electrochemical properties of graphene composites [J]. Inorganic Chemicals Industry, 2022, 54(12): 68-73. |
| [8] | WANG Wei,LIU Wei,WU Yang,YANG Shenshen. Research progress on molybdenum disulfide-based anode materials for lithium-ion batteries [J]. Inorganic Chemicals Industry, 2022, 54(10): 87-95. |
| [9] | Jian Mengqi,Zhang Kun,Xie Xin,Chen Xiyong. Research progress of LiMnPO4 cathode material for lithium ion batteries [J]. Inorganic Chemicals Industry, 2021, 53(9): 18-23. |
| [10] | Bao Kejie,Lu lingran. Study on preparation and performance of negative electrode materials for batteries of new energy vehicles [J]. Inorganic Chemicals Industry, 2021, 53(3): 54-59. |
| [11] | CHENG Liqun,ZUO Fushan. Study on preparation and properties of magnesium free hydrogen storage alloys for new energy vehicle batteries [J]. Inorganic Chemicals Industry, 2021, 53(11): 71-76. |
| [12] | Fan Qingke,Meng Qinghua,Luo Fengyu. Study on preparation and properties of cathode materials for vehicle lithium battery [J]. Inorganic Chemicals Industry, 2021, 53(1): 44-49. |
| [13] | Guo Ju,Jia Shuangzhu. Study of the method for the preparation of spherical-like and low sulfur FePO4 [J]. Inorganic Chemicals Industry, 2020, 52(5): 31-34. |
| [14] | Li Tong,Shi Yunbin,Liu Qingbin,He Shuang. Structure and electrochemical properties of cathode materials for new energy automotive lithium batteries doped and modified by Se [J]. Inorganic Chemicals Industry, 2020, 52(2): 17-22. |
| [15] | YANG Jian-Wen, YOU Hai-Ping, DENG Xing-Shen, PAN Xiao-Jin, YANG Gui-Jun, ZHANG Ling-Zhi. Preparation and electrochemical performances of chiral porous sodium zinc phosphate as anode material [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(2): 75-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||