Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (1): 33-39.doi: 10.19964/j.issn.1006-4990.2023-0302
• Research & Development • Previous Articles Next Articles
Study on solid-liquid phase equilibrium of ternary system of Li+, Na+∥Cl--H2O at different temperatures
WANG Yanfei1,2( ), YANG Chaofan1,2, CAO Di1,2, XU Shijie1,2(
), YANG Chaofan1,2, CAO Di1,2, XU Shijie1,2( )
)
- 1. Tianjin Key Laboratory of Brine Chemical Engineering and Ecological Utilization of Resources, Tianjin 300457, China
2. College of Chemical Engineering and Materials, Tianjin University of Science and Technology, Tianjin 300457, China
-
Received:2023-06-02Online:2024-01-10Published:2024-01-18 -
Contact:WANG Yanfei, XU Shijie E-mail:wangyanfei@tust.edu.cn;xushijie@tust.edu.cn
CLC Number:
Cite this article
WANG Yanfei, YANG Chaofan, CAO Di, XU Shijie. Study on solid-liquid phase equilibrium of ternary system of Li+, Na+∥Cl--H2O at different temperatures[J]. Inorganic Chemicals Industry, 2024, 56(1): 33-39.
share this article
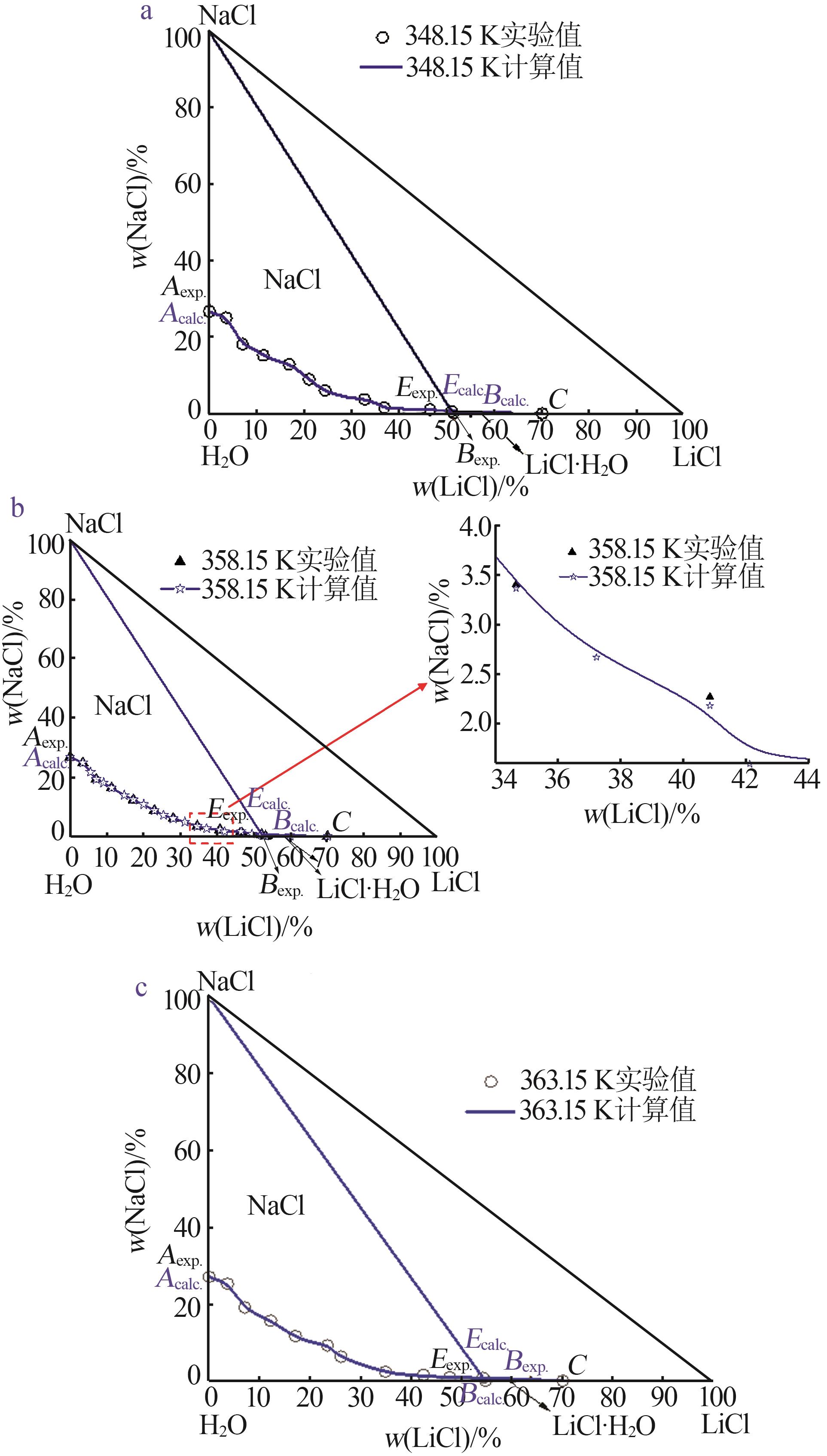
Table 1
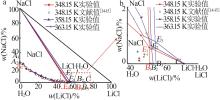
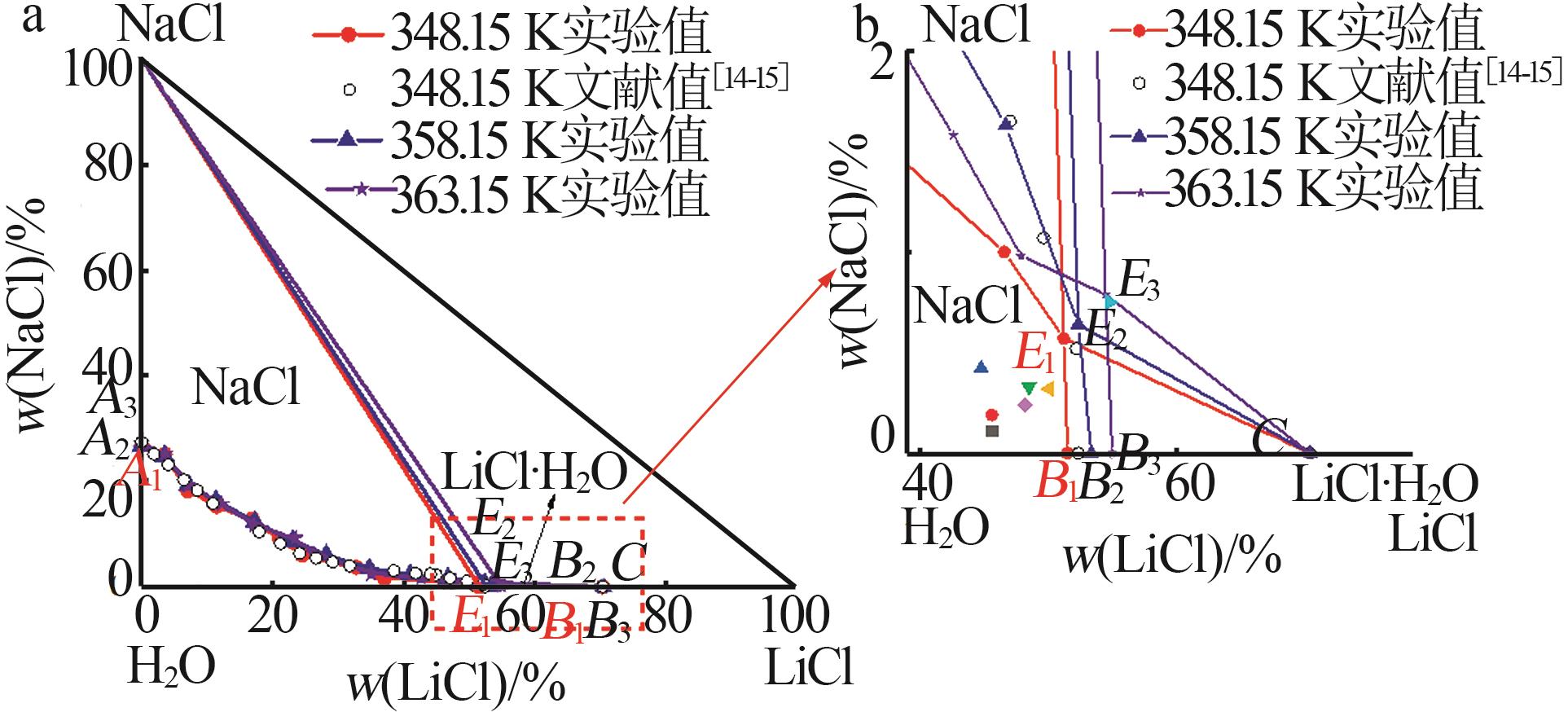
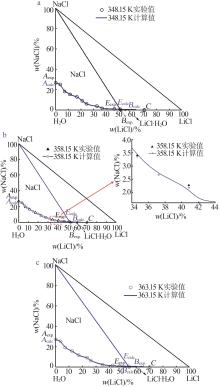
Experimental and calculated phase equilibrium data of ternary system of Li+,Na+ ∥Cl--H2O at 348.15~363.15 Ka"
| 液相组分实验值/%±标准不确定度/% | 液相组分计算值/% | 相对偏差(RD)/% | 平衡 固相b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| w(LiCl) | w(NaCl) | w(H2O) | w(LiCl) | w(NaCl) | w(H2O) | w(LiCl) | w(NaCl) | w(H2O) | ||||
| T=348.15 K | ||||||||||||
| 0.00 | 26.72±0.12 | 73.28±0.21 | 0.00 | 26.62 | 73.38 | 0.00 | -0.37 | 0.14 | N | |||
| 3.56±0.39 | 24.98±0.75 | 71.46±0.36 | 3.56 | 25.02 | 71.42 | 0.00 | 0.16 | -0.06 | N | |||
| 7.00±0.56 | 18.16±0.29 | 74.84±0.86 | 7.00 | 18.15 | 74.85 | 0.00 | -0.06 | 0.01 | N | |||
| 11.37±0.18 | 15.24±0.22 | 73.39±0.04 | 11.37 | 15.23 | 73.40 | 0.00 | -0.07 | 0.01 | N | |||
| 16.85±0.45 | 12.87±0.21 | 70.27±0.28 | 16.85 | 12.92 | 70.23 | 0.00 | 0.39 | -0.06 | N | |||
| 21.04±0.63 | 8.97±0.20 | 70.00±0.43 | 21.04 | 8.91 | 70.05 | 0.00 | -0.67 | 0.07 | N | |||
| 24.44±0.50 | 5.98±0.86 | 69.58±0.36 | 24.44 | 5.96 | 69.60 | 0.00 | -0.33 | 0.03 | N | |||
| 32.72±0.43 | 3.68±0.41 | 63.61±0.19 | 32.72 | 3.67 | 63.61 | 0.00 | -0.27 | 0.00 | N | |||
| 36.97±0.01 | 1.55±0.39 | 61.48±0.38 | 36.97 | 1.55 | 61.48 | 0.00 | 0.00 | 0.00 | N | |||
| 46.50±0.10 | 1.00±0.07 | 52.50±0.13 | 46.50 | 1.00 | 52.50 | 0.00 | 0.00 | 0.00 | N | |||
| 51.11±0.54 | 0.57±0.02 | 48.31±0.56 | 51.11 | 0.56 | 48.33 | 0.00 | -1.75 | 0.04 | N+L | |||
| 51.41±0.11 | 0.00 | 48.59±0.23 | 51.41 | 0.00 | 48.59 | 0.00 | 0.00 | 0.00 | L | |||
| T=358.15 K | ||||||||||||
| 0.00 | 26.85±0.17 | 73.15±0.11 | 0.00 | 26.83 | 73.17 | 0.00 | -0.07 | 0.03 | N | |||
| 3.48±0.01 | 24.80±0.03 | 71.72±0.10 | 3.48 | 24.82 | 71.70 | 0.00 | 0.08 | -0.03 | N | |||
| 7.11±0.02 | 19.22±0.81 | 73.67±0.57 | 7.11 | 19.22 | 73.67 | 0.00 | 0.00 | 0.00 | N | |||
| 11.31±0.04 | 16.38±0.71 | 72.31±1.13 | 11.31 | 16.38 | 72.31 | 0.00 | 0.00 | 0.00 | N | |||
| 17.21±0.03 | 12.27±0.36 | 70.52±0.03 | 17.21 | 12.38 | 70.41 | 0.00 | 0.90 | -0.16 | N | |||
| 22.95±0.60 | 8.73±0.14 | 68.32±1.73 | 22.95 | 8.69 | 68.36 | 0.00 | -0.46 | 0.06 | N | |||
| 28.09±0.05 | 6.00±0.17 | 65.91±0.63 | 28.09 | 6.00 | 65.91 | 0.00 | 0.00 | 0.00 | N | |||
| 34.67±0.78 | 3.41±0.08 | 61.92±1.89 | 34.67 | 3.37 | 61.96 | 0.00 | -1.17 | 0.06 | N | |||
| 40.86±0.04 | 2.27±0.49 | 56.87±0.91 | 40.86 | 2.18 | 57.36 | 0.00 | -3.96 | 0.16 | N | |||
| 46.61±0.07 | 1.63±0.18 | 51.76±0.72 | 46.61 | 1.68 | 51.61 | 0.00 | 3.07 | -0.10 | N | |||
| 52.23±0.40 | 0.64±0.09 | 47.13±0.91 | 52.23 | 0.61 | 47.16 | 0.00 | -4.69 | 0.06 | N+L | |||
| 53.25±0.14 | 0.00 | 46.75±0.27 | 53.25 | 0.00 | 46.75 | 0.00 | 0.00 | 0.00 | L | |||
| T=363.15 K | ||||||||||||
| 0.00 | 27.10±0.27 | 72.90±0.11 | 0.00 | 27.11 | 72.89 | 0.00 | 0.04 | -0.01 | N | |||
| 3.66±0.13 | 25.31±0.58 | 71.03±0.45 | 3.66 | 25.34 | 71.00 | 0.00 | 0.12 | -0.04 | N | |||
| 7.05±0.14 | 19.13±0.95 | 73.82±0.81 | 7.05 | 19.13 | 73.82 | 0.00 | 0.00 | 0.01 | N | |||
| 12.22±0.67 | 15.66±0.76 | 72.13±1.43 | 12.22 | 15.68 | 72.10 | 0.00 | 0.13 | -0.04 | N | |||
| 23.46±1.12 | 9.21±0.27 | 67.33±0.85 | 23.46 | 9.22 | 67.32 | 0.00 | 0.11 | -0.02 | N | |||
| 26.20±0.76 | 6.28±0.11 | 67.52±0.66 | 26.20 | 6.29 | 67.51 | 0.00 | 0.16 | 0.02 | N | |||
| 34.97±0.39 | 2.39±0.05 | 62.64±0.34 | 34.97 | 2.38 | 62.65 | 0.00 | -0.42 | 0.03 | N | |||
| 47.80±0.97 | 0.98±0.26 | 51.22±1.23 | 47.80 | 0.98 | 51.22 | 0.00 | 0.00 | 0.00 | N | |||
| 54.35±0.20 | 0.79±0.01 | 44.86±0.22 | 54.35 | 0.78 | 44.87 | 0.00 | -1.27 | 0.02 | N+L | |||
| 54.87±0.10 | 0.00 | 45.13±0.61 | 54.87 | 0.00 | 45.13 | 0.00 | 0.00 | 0.00 | L | |||
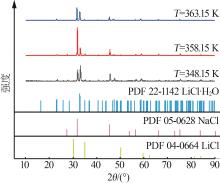
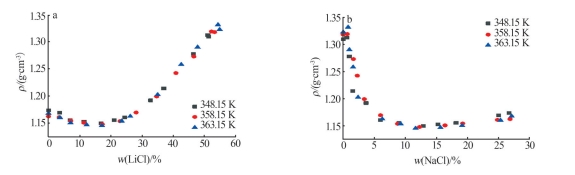
Table 4
Densities of saturated solution of ternary systemof Li+,Na+ ∥Cl--H2O at 348.15,358.15,363.15 K"
| 编号 | 饱和溶液密度/(g∙cm-3) | 相对偏差(RD)/% | ||||
|---|---|---|---|---|---|---|
| ρe±标准不确定度a | ρc(1) | ρc(2) | RD(1) | RD(2) | ||
| T=348.15 K | ||||||
| 1,A1 | 1.173 5±0.001 3 | 1.163 8 | 1.171 7 | -0.83 | -0.15 | |
| 2 | 1.169 0±0.002 6 | 1.173 8 | 1.171 0 | 0.41 | 0.17 | |
| 3 | 1.155 3±0.001 9 | 1.144 0 | 1.155 4 | -0.98 | 0.01 | |
| 4 | 1.152 5±0.000 9 | 1.150 1 | 1.152 4 | -0.21 | -0.01 | |
| 5 | 1.149 6±0.004 1 | 1.167 8 | 1.149 1 | 1.58 | -0.04 | |
| 6 | 1.155 2±0.003 2 | 1.165 2 | 1.155 2 | 0.86 | 0.00 | |
| 7 | 1.160 8±0.002 9 | 1.164 5 | 1.161 6 | 0.32 | 0.07 | |
| 8 | 1.192 1±0.003 8 | 1.201 8 | 1.193 8 | 0.82 | 0.15 | |
| 9 | 1.214 2±0.004 3 | 1.213 8 | 1.212 3 | -0.04 | -0.15 | |
| 10 | 1.278 0±0.002 7 | 1.276 3 | 1.276 1 | -0.13 | -0.14 | |
| 11,E1 | 1.312 9±0.007 8 | 1.306 3 | 1.310 5 | -0.51 | -0.19 | |
| 12,B1 | 1.309 9±0.001 6 | 1.303 5 | 1.313 8 | -0.49 | 0.30 | |
| T=358.15 K | ||||||
| 1,A2 | 1.162 3±0.001 7 | 1.155 3 | 1.159 5 | -0.60 | -0.25 | |
| 2 | 1.160 9±0.001 1 | 1.162 6 | 1.164 3 | 0.15 | 0.29 | |
| 3 | 1.154 3±0.001 7 | 1.144 2 | 1.154 3 | -0.88 | 0.00 | |
| 4 | 1.150 7±0.003 8 | 1.150 2 | 1.151 2 | -0.05 | 0.04 | |
| 5 | 1.147 8±0.002 8 | 1.157 7 | 1.146 4 | 0.87 | -0.12 | |
| 6 | 1.153 6±0.001 8 | 1.168 7 | 1.153 8 | 1.31 | 0.02 | |
| 7 | 1.169 6±0.002 2 | 1.182 0 | 1.171 6 | 1.06 | 0.17 | |
| 8 | 1.199 3±0.003 2 | 1.206 3 | 1.202 1 | 0.58 | 0.24 | |
| 9 | 1.242 6±0.003 7 | 1.240 2 | 1.237 2 | -0.19 | -0.44 | |
| 10 | 1.273 2±0.012 1 | 1.276 0 | 1.275 0 | 0.22 | 0.14 | |
| 11,E2 | 1.319 6±0.002 9 | 1.308 9 | 1.317 3 | -0.82 | -0.18 | |
| 12,B2 | 1.318 6±0.005 6 | 1.311 0 | 1.322 3 | -0.58 | 0.28 | |
| T=363.15 K | ||||||
| 1,A3 | 1.167 8±0.002 5 | 1.155 4 | 1.160 6 | -1.07 | -0.62 | |
| 2 | 1.160 2±0.002 3 | 1.166 2 | 1.168 5 | 0.52 | 0.72 | |
| 3 | 1.150 3±0.001 0 | 1.141 6 | 1.148 3 | -0.76 | -0.18 | |
| 4 | 1.146 7±0.000 8 | 1.149 5 | 1.146 8 | 0.25 | 0.01 | |
| 5 | 1.145 3±0.005 4 | 1.151 6 | 1.145 9 | 0.56 | 0.05 | |
| 6 | 1.153 8±0.001 3 | 1.175 6 | 1.152 0 | 1.90 | -0.15 | |
| 7 | 1.162 6±0.004 2 | 1.171 5 | 1.167 9 | 0.76 | 0.45 | |
| 8 | 1.202 7±0.003 0 | 1.201 3 | 1.205 9 | -0.12 | 0.27 | |
| 9 | 1.258 4±0.012 9 | 1.248 5 | 1.252 5 | -0.78 | -0.47 | |
| 10 | 1.290 6±0.006 6 | 1.282 0 | 1.286 4 | -0.66 | -0.32 | |
| 11,E3 | 1.332 2±0.001 1 | 1.330 0 | 1.327 7 | -0.17 | -0.33 | |
| 12,B3 | 1.323 3±0.005 5 | 1.327 0 | 1.332 7 | 0.28 | 0.71 | |
Table 5
Fitting parameters and R2 with Eq.(9) of ternary system of Li+,Na+∥Cl--H2O at 348.15,358.15,363.15 K"
| T/K | 拟合常数 | 相关系数R2 | 最大离子强度 Imax/(mol∙kg-1) | |||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F/10-7 | |||
| 348.15 | 0.024 37 | 0.077 14 | -0.003 38 | -0.002 16 | -0.018 25 | 1.533 09 | 0.999 09 | 25.157 7 |
| 358.15 | 0.023 48 | 0.071 16 | -0.002 23 | -0.001 99 | -0.016 27 | 2.309 73 | 0.998 24 | 26.868 3 |
| 363.15 | 0.027 00 | 0.055 65 | -0.002 14 | -0.002 65 | -0.009 89 | -1.928 68 | 0.994 41 | 28.874 8 |
Table 6
Apparent molar volumes of ternary system of LiCl (1)-NaCl (2)-H2O at 348.15,358.15,363.15 K"
| 编号 | 348.15 K | 358.15 K | 363.15 K | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
m1/ (mol∙kg-1) | m2/ (mol∙kg-1) | Vφ / (cm3∙mol-1) | m1/ (mol∙kg-1) | m2/ (mol∙kg-1) | Vφ / (cm3∙mol-1) | m1/ (mol∙kg-1) | m2/ (mol∙kg-1) | Vφ / (cm3∙mol-1) | |||
| 1 | — | 6.238 1 | 21.967 4 | — | 6.281 2 | 22.886 8 | — | 6.360 2 | 21.802 0 | ||
| 2 | 1.176 3 | 5.980 4 | 23.930 0 | 1.143 1 | 5.916 2 | 23.879 2 | 1.214 8 | 6.097 2 | 24.270 7 | ||
| 3 | 2.207 0 | 4.151 7 | 20.565 4 | 2.276 6 | 4.462 8 | 21.289 9 | 2.253 6 | 4.435 3 | 21.193 4 | ||
| 4 | 3.653 5 | 3.553 1 | 21.710 7 | 3.690 9 | 3.874 8 | 22.387 2 | 3.995 6 | 3.714 4 | 22.458 3 | ||
| 5 | 5.656 8 | 3.134 7 | 24.119 7 | 5.757 6 | 2.975 9 | 23.244 0 | 5.668 2 | 2.796 2 | 22.416 6 | ||
| 6 | 7.088 8 | 2.192 0 | 22.722 4 | 7.925 3 | 2.185 5 | 23.381 9 | 8.217 3 | 2.340 7 | 23.800 6 | ||
| 7 | 8.286 9 | 1.471 0 | 21.767 7 | 10.055 0 | 1.557 5 | 22.808 7 | 9.152 9 | 1.591 8 | 22.145 8 | ||
| 8 | 12.133 2 | 0.989 1 | 22.334 2 | 13.208 8 | 0.940 9 | 22.206 4 | 13.170 4 | 0.654 3 | 21.087 6 | ||
| 9 | 14.185 7 | 0.432 0 | 21.469 6 | 16.949 4 | 0.682 9 | 21.706 5 | 17.969 4 | 0.484 7 | 20.950 5 | ||
| 10 | 20.893 7 | 0.325 9 | 21.900 0 | 21.245 6 | 0.538 9 | 22.271 2 | 22.014 5 | 0.327 3 | 21.346 1 | ||
| 11 | 24.954 7 | 0.203 0 | 21.888 4 | 26.139 6 | 0.231 8 | 21.818 7 | 28.575 4 | 0.299 5 | 22.068 2 | ||
| 12 | 24.961 2 | 0.000 0 | 21.852 2 | 26.868 3 | 0.000 0 | 21.951 7 | 28.682 6 | 0.000 0 | 22.265 6 | ||
| 1 | 况新亮,刘垂祥,熊朋.锂离子电池产业分析及市场展望[J].无机盐工业,2022,54(8):12-19,32. |
| KUANG Xinliang, LIU Chuixiang, XIONG Peng.Industry analysis and market prospect of lithium ion battery[J].Inorganic Chemicals Industry,2022,54(8):12-19,32. | |
| 2 | YU Xiaoping, FAN Xuebing, GUO Yafei,et al.Recovery of lithium from underground brine by multistage centrifugal extraction using tri-isobutyl phosphate[J].Separation and Purification Technology,2019,211:790-798. |
| 3 | WANG Shulei, ZHENG Shili, WANG Zheming,et al.Superior lithium adsorption and required magnetic separation behavior of iron-doped lithium ion-sieves[J].Chemical Engineering Journal,2018,332:160-168. |
| 4 | 王彦飞,胡佳琪,卫丽莎,等.添加剂对碳酸锂结晶的影响[J].无机盐工业,2018,50(6):42-46. |
| WANG Yanfei, HU Jiaqi, WEI Lisha,et al.Effect of additives on lithium carbonate crystallization[J].Inorganic Chemicals Industry,2018,50(6):42-46. | |
| 5 | 王彦飞,李亚楠,胡佳琪,等.去除氯化锂中氯化钠的研究进展[J].无机盐工业,2018,50(2):13-15. |
| WANG Yanfei, LI Yanan, HU Jiaqi,et al.Progress in separating sodium chloride from lithium chloride[J].Inorganic Chemicals Industry,2018,50(2):13-15. | |
| 6 | SMITS A, ELGERSMA J, HARDENBERG M M E.A critical mixing point in the solid phase of the system NaCl-LiCl[J].Recueil des Travaux Chimiques des Pays-Bas,1924,43(9):671-676. |
| 7 | FARELO F, FERNANDES C, AVELINO A.Solubilities for six ternary systems:NaCl+NH4Cl+H2O,KCl+NH4Cl+H2O,NaCl+LiCl+H2O,KCl+LiCl+H2O,NaCl+AlCl3+H2O,and KCl+AlCl3+H2O at T=(298 to 333) K[J].ChemInform,2005,36(40):200540019. |
| 8 | WANG Shiqiang, GUO Yafei, LIU Dongfang,et al.Phase equilibria in system LiCl-NaCl-H2O at 308 and 348 K[J].Russian Journal of Physical Chemistry A,2016,90(13):2532-2537. |
| 9 | YANG Haitang, ZENG Dewen, CHEN Yifeng,et al.Revisiting the thermodynamic properties of the LiCl-NaCl-KCl-H2O quaternary and its sub-ternary systems at 298.15 K[J].Calphad,2015,50:161-169. |
| 10 | RATHGEBER C, SCHMIT H, HIEBLER S,et al.Application of the modified BET model to concentrated salt solutions with relatively high water activities:Predicting solubility phase diagrams of NaCl+H2O,NaCl+LiCl+H2O,and NaCl+CaCl2+H2O[J].Calp- had,2019,66:101633. |
| 11 | LOVERA J, TÍJARO-ROJAS R, MERUANE G,et al.Prediction of solubilities in the system Li++Na++K++Cl–+H2O at 25 ℃ and industrial process of refining muriate of potash[J].Journal of Chemical & Engineering Data,2019,64(5):2027-2035. |
| 12 | CUI Ruizhi.Solubility measurement and prediction of phase equilibria in the quaternary system LiCl+NaCl+KCl+H2O and ternary subsystem LiCl+NaCl+H2O at 288.15 K[J].Chinese Journal of Chemical Engineering,2020,28(8):2137-2141. |
| 13 | LI Cheng, ZHAO Bin, WANG Shuo,et al.Phase diagrams of the quinary system K+,NH4 +,Mg2+∥SO4 2-,Cl--H2O at 273.15 K and 298.15 K and their application[J].Fluid Phase Equilibria,2019,499:112238. |
| 14 | LI Cheng, WU Jingxue, GUO Hongfei,et al.Phase diagrams of the quaternary system K+,NH4 +∥Cl-,SO4 2--H2O at 273.15 K and their application[J].The Journal of Chemical Thermodynamics,2019,134:136-145. |
| 15 | ZHANG Jijun, REN Mengyuan, LI Dongchan,et al.Solid-liquid phase equilibrium in the quaternary system(LiCl+NaCl+KCl+H2O) at 288.15 K[J].Russian Journal of Physical Chemistry A,2020,94(8):1565-1572. |
| 16 | VOIGT H, VOIGT W.Solubility isotherm of the system LiNO3-KNO3-H2O at 373 K[J].Monatshefte Für Chemie-Chemical Monthly,2018,149(2):283-288. |
| 17 | MARCUS Y.BET modeling of solid-liquid phase diagrams of co-mmon ion binary salt hydrate mixtures.I.The BET parameters[J].Journal of Solution Chemistry,2005,34(3):297-306. |
| 18 | NGUYEN M T H, TICHACEK O, MARTINEZ-SEARA H,et al.Resolving the equal number density puzzle:Molecular picture from simulations of LiCl(aq) and NaCl(aq)[J].The Journal of Physical Chemistry B,2021,125(12):3153-3162. |
| 19 | HASSEINE A, MENIAI A H, KORICHI M.Salting-out effect of single salts NaCl and KCl on the LLE of the systems(water+toluene+acetone),(water+cyclohexane+2-propanol) and(water+xylene+methanol)[J].Desalination,2009,242(1/2/3):264-276. |
| 20 | 申屠雁明,于养信,李以圭.混合电解质溶液的密度和表观摩尔体积的研究[J].清华大学学报(自然科学版),1993,33(6):61-72. |
| SHENTU Yanming, YU Yangxin, LI Yigui.Study on densities and apparent molal volumes of mixed electrolyte aqueous solutions[J].Journal of Tsinghua University(Science and Technology),1993,33(6):61-72. | |
| 21 | 林联君,房春晖,房艳,等.一个预测溶液密度的新模型[J].盐湖研究,2006,14(2):56-61. |
| LIN Lianjun, FANG Chunhui, FANG Yan,et al.A new model for predicting density of electrolyte solutions[J].Journal of Salt Lake Research,2006,14(2):56-61. |
| [1] | YAO Jiankang, HU Shuozhen, NIU Dongfang, WU Jianping, ZHANG Xinsheng. Study on electrochemical treatment of sodium chloride organic waste salt in spice industry [J]. Inorganic Chemicals Industry, 2024, 56(3): 105-115. |
| [2] | YANG Shuiyan, YANG Mingxia, XIN Wanwan. Study on preparation and properties of high⁃purity lithium 2-trifluoromethyl-4,5-dicyanoimidazole [J]. Inorganic Chemicals Industry, 2024, 56(2): 74-79. |
| [3] | SHEN Jiaqi,YAN Shenghu,ZHANG Yue,LIU Jianwu,SHEN Jiefa. Study on intensification of gas-liquid reaction process for sodium chloride waste salt to ammonium alkali [J]. Inorganic Chemicals Industry, 2022, 54(12): 99-105. |
| [4] | Cao Liqiong,Zhang Xiaoxi,Wu Lixiang,Cheng Huaigang,Cheng Fangqin. Experimental investigation of floating phenomenon of carnalliti NaCl in direct flotation process of KCl [J]. Inorganic Chemicals Industry, 2020, 52(7): 26-29. |
| [5] | Chen Jie,Chen Xia. Crystallization process optimization for preparation of lithium carbonate [J]. Inorganic Chemicals Industry, 2019, 51(8): 29-32. |
| [6] | CHENG Huai-De, MA Hai-Zhou, ZHANG Zhi-Hong. Preliminary study on effect of high-magnesium solution on carnallite decomposition [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(1): 21-. |
| [7] | ZHAO Jing, CHENG Wen-Ting, CAO Qin-Bo, CHENG Fang-Qin. Behaviors of sodium chloride floated with potassium chloride in direct flotation process [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(6): 33-. |
| [8] | XU Jing-Feng, XIE Ting, CAO Zi-Ying. New preparation method of sodium sulphate-hydrogen peroxide-sodium chloride adduct [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(12): 36-. |
| [9] | Shi Xiaoping;Hu Jianxun;Liu Changsong;Wei Feng;Hu Baisong. Comprehensive treatment for mother solution of sodium bicarbonate by double decomposition method [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(8): 0-0. |
| [10] | Tao Yuhong;Xu Guocai;Li Deji. Preparation and surface analysis of superfine sodium chloride powder with hollow structure [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(7): 0-0. |
| [11] | Ding Xiuping;Sun Bai;Shi Lijie;Yang Haitang;Song Pengsheng. Study on phase equilibria in NaCl-SrCl2-H2O ternary system at 25 ℃ [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(6): 0-0. |
| [12] | Yang Jimin;Xu Guanghua;Wang Yan. Study on phase equilibrium of lithium chloride-ethanol-water ternary system at 30 ℃ [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(5): 0-0. |
| [13] | Zeng Bo;Yang Yan;Li Haili. Study on preparation of sodium dihydrogen phosphate by extraction method [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(4): 0-0. |
| [14] | Tang Xiuhua. Preparation of sodium sulphate-hydrogen peroxide-sodium chloride adduct [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(2): 0-0. |
| [15] | Hu Zhongyu;Luo Daocheng. Study on decoloring spectrophotometric determination of micro bromate ion [J]. INORGANICCHEMICALSINDUSTRY, 2009, 0(7): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||