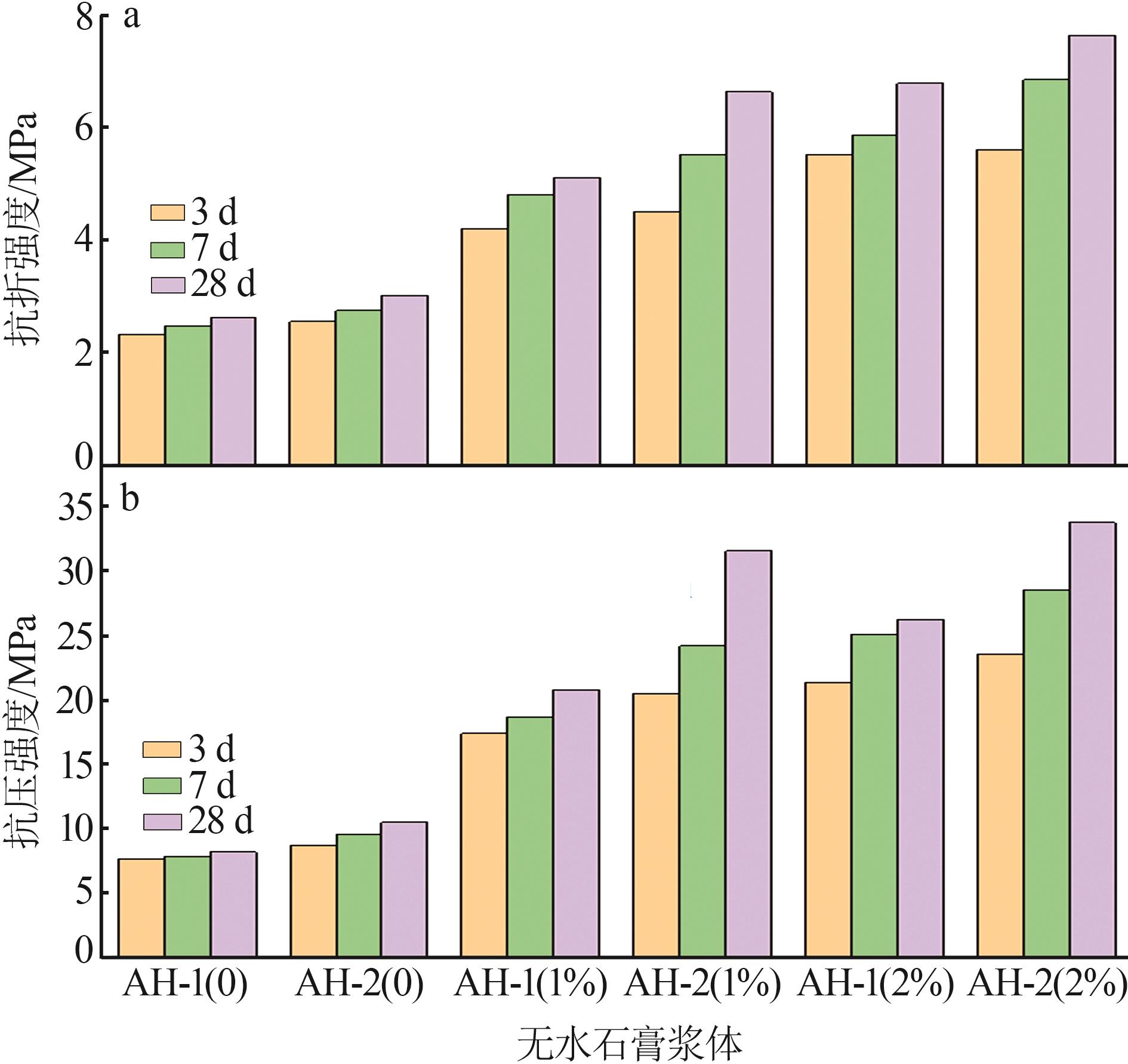
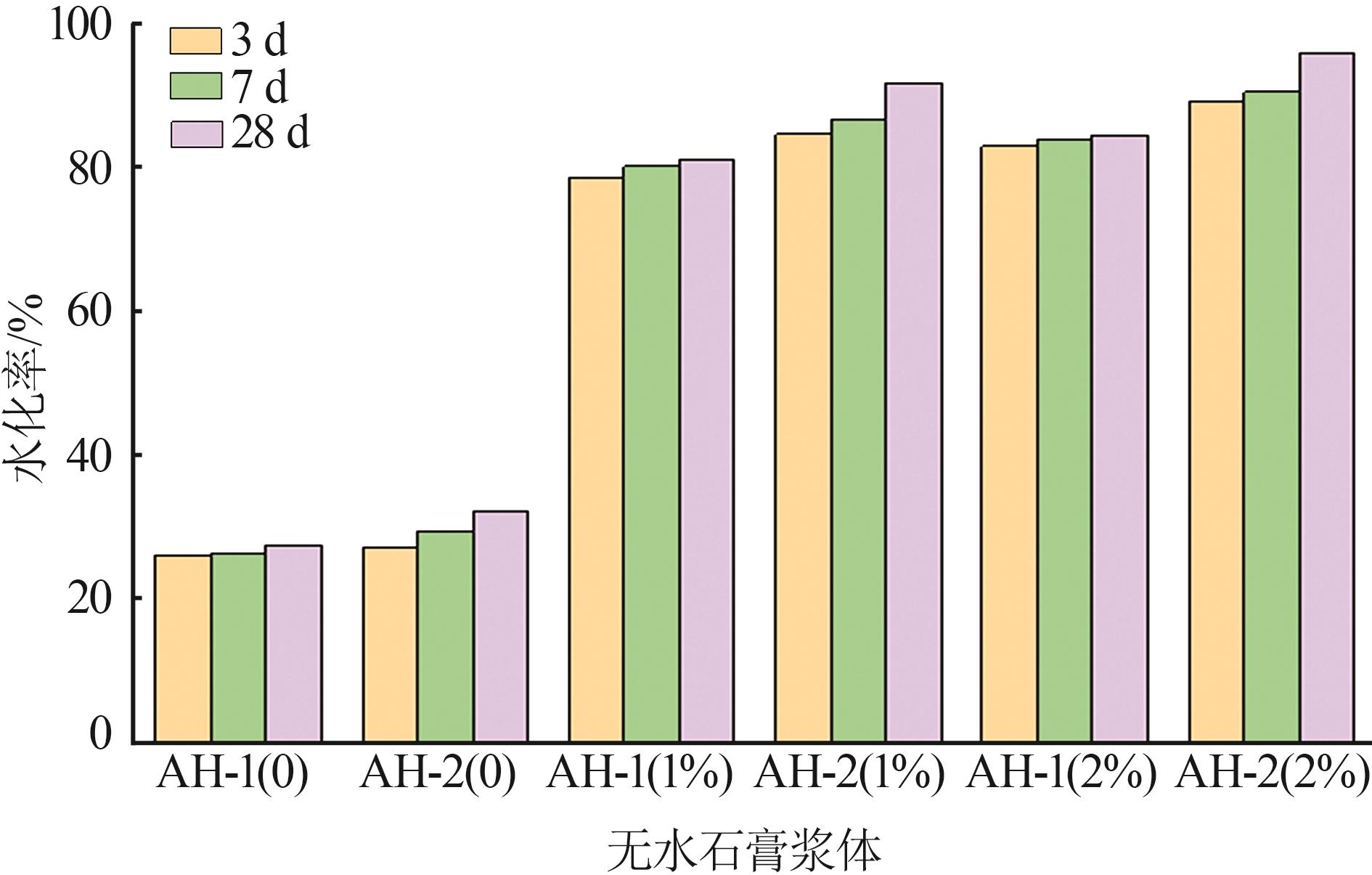
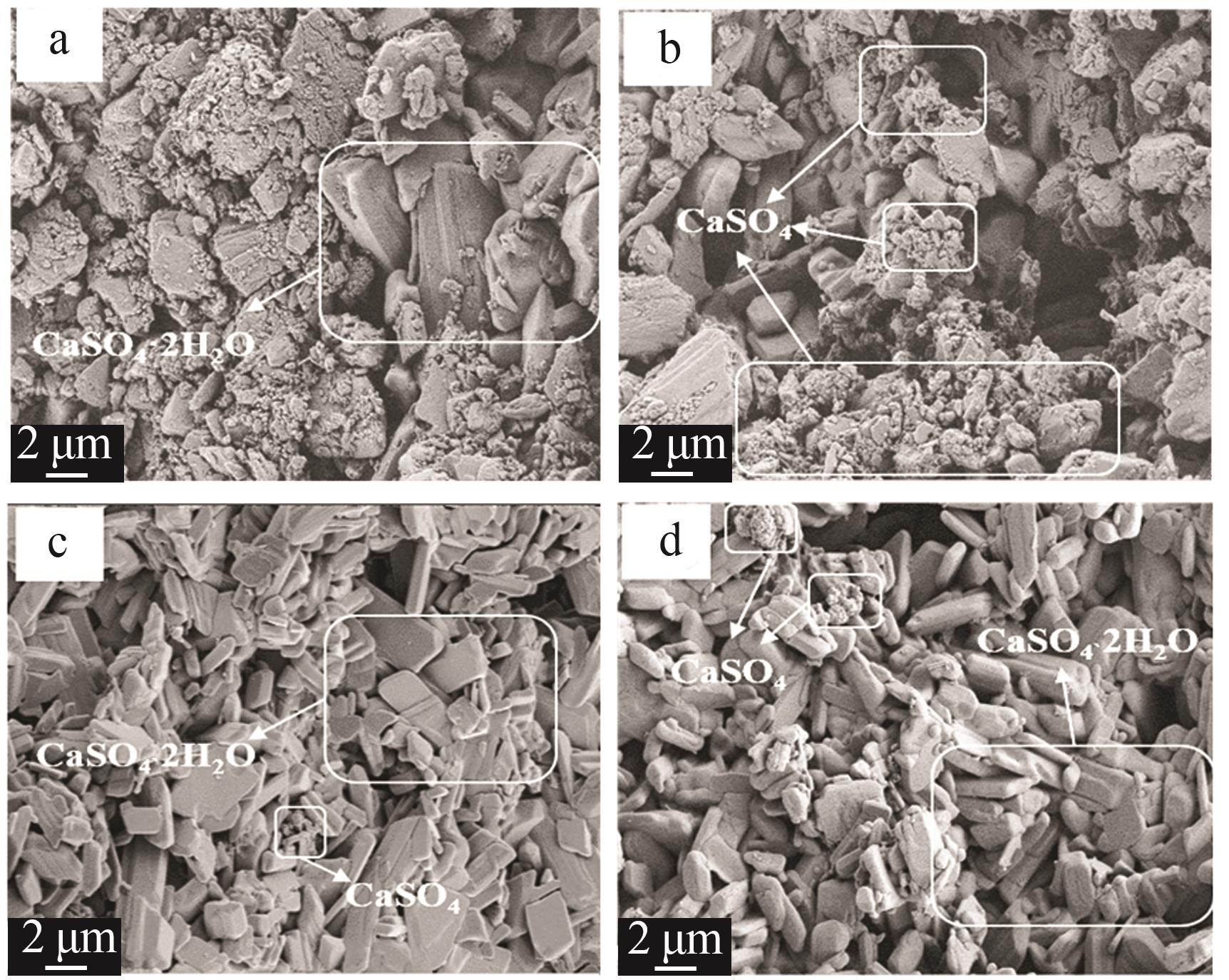
| [1] |
WANG Peixiong, GONG Xiaomei, DING Jiaqi, CAO Hong.
Effect of crystal modifier on preparation of α-hemihydrates gypsum from industrial gypsum
[J]. Inorganic Chemicals Industry, 2024, 56(4): 112-117.
|
| [2] |
YU Zhou, HE Zhaoyi, TANG Liang, HE Sheng, XIAO Haixin, XIAO Yixun.
Study on preparation and microscopic properties of typical sulfate solid waste composite cementitious materials
[J]. Inorganic Chemicals Industry, 2024, 56(4): 90-97.
|
| [3] |
CHEN Feng, FENG Kang, LI Ming, SHEN Haojie, TIAN Chengtao, TANG Yuan, LI Zhili, HE Dongsheng.
Application of organically modified calcium sulfate whiskers in asphalt modification
[J]. Inorganic Chemicals Industry, 2024, 56(3): 125-130.
|
| [4] |
WANG Ruting, ZHAO Xiaorong, HUANG Xuquan, WANG Haojie, XUE Fei, CAI Jiawei.
Research on preparation and early performance of mixed phase phosphogypsum-based cementing materials
[J]. Inorganic Chemicals Industry, 2024, 56(3): 98-104.
|
| [5] |
WANG Yanyu, GU Shouyu, HOU Cuihong, JING Hongquan, GUAN Hongling, ZHANG Hui.
Sulfur escape and slag physical phase analysis by carbon thermal reduction melting based on phosphogypsum ingredients
[J]. Inorganic Chemicals Industry, 2024, 56(2): 86-94.
|
| [6] |
DENG Hua, HOU Shuomin, LI Zhongjun, XU Gang, CHI Ru′an, XI Benjun.
Current situation and prospect of comprehensive utilization of phosphogypsum
[J]. Inorganic Chemicals Industry, 2024, 56(1): 1-8.
|
| [7] |
XIA Guiying, YANG Liuchun, YUAN Zhiye.
Study on direct leaching of rare earth elements from phosphogypsum with sulfuric acid
[J]. Inorganic Chemicals Industry, 2024, 56(1): 107-113.
|
| [8] |
XIANG Mengqi, MENG Hua, WANG Ye, MENG Xianzhang, BAI Yuhang, WANG Yujunyao, ZHANG Yidan.
Study on kinetic of iron leaching from titanium gypsum and its cyclic acid leaching process
[J]. Inorganic Chemicals Industry, 2024, 56(1): 114-120.
|
| [9] |
CUI Gengyin, XIE Lang, LU Yuexian, KONG Dewen, WANG Lingling.
Optimization of mechanical properties of basalt fiber reinforced phosphogypsum-based composites based on RSM
[J]. Inorganic Chemicals Industry, 2023, 55(8): 116-123.
|
| [10] |
LI Heng, ZHANG Hui, ZI Xuemin.
Analysis on calcination process progress of phosphogypsum
[J]. Inorganic Chemicals Industry, 2023, 55(6): 27-35.
|
| [11] |
ZHANG Lei, LI Meng, XIANG Wenguo, HU Jun, CHEN Shiyi, DUAN Lunbo.
Feasibility analysis of calcination and decomposition process of phosphogypsum in circulating fluidized bed
[J]. Inorganic Chemicals Industry, 2023, 55(6): 85-91.
|
| [12] |
XU Li, FANG Keneng, BI Yongxiang, YANG Min, CHEN Qianlin.
Preparation of modified granular-like CaSO4 and its application in polyvinyl chloride
[J]. Inorganic Chemicals Industry, 2023, 55(3): 104-112.
|
| [13] |
GUO Shuang, XING Dongxian, GUO Xiao, JI Xiaojie, TANG Jianwei, HUA Quanxian, WANG Baoming, LIU Yong.
Study on preparation and modification of phosphogypsum-based architectural gypsum
[J]. Inorganic Chemicals Industry, 2023, 55(12): 102-110.
|
| [14] |
LIU Chao, FAN Chuigang, LIU Runguo, YU Dongxue, LI Songgeng.
Preparation of α-hemihydrate gypsum from phosphogypsum by flotation and atmospheric trans-crystallization in solution
[J]. Inorganic Chemicals Industry, 2023, 55(11): 107-114.
|
| [15] |
SUN Zhigao, WU Yuan, YANG Xingchun, ZHANG Dongliang, WANG Mitang.
Preparation and properties of high-magnesium nickel slag-fly ash based geopolymer
[J]. Inorganic Chemicals Industry, 2023, 55(11): 139-146.
|
 ), YANG Xuejiao3, GUO Xudong3, YANG Lin1,2(
), YANG Xuejiao3, GUO Xudong3, YANG Lin1,2( )
)