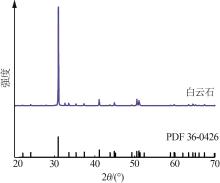
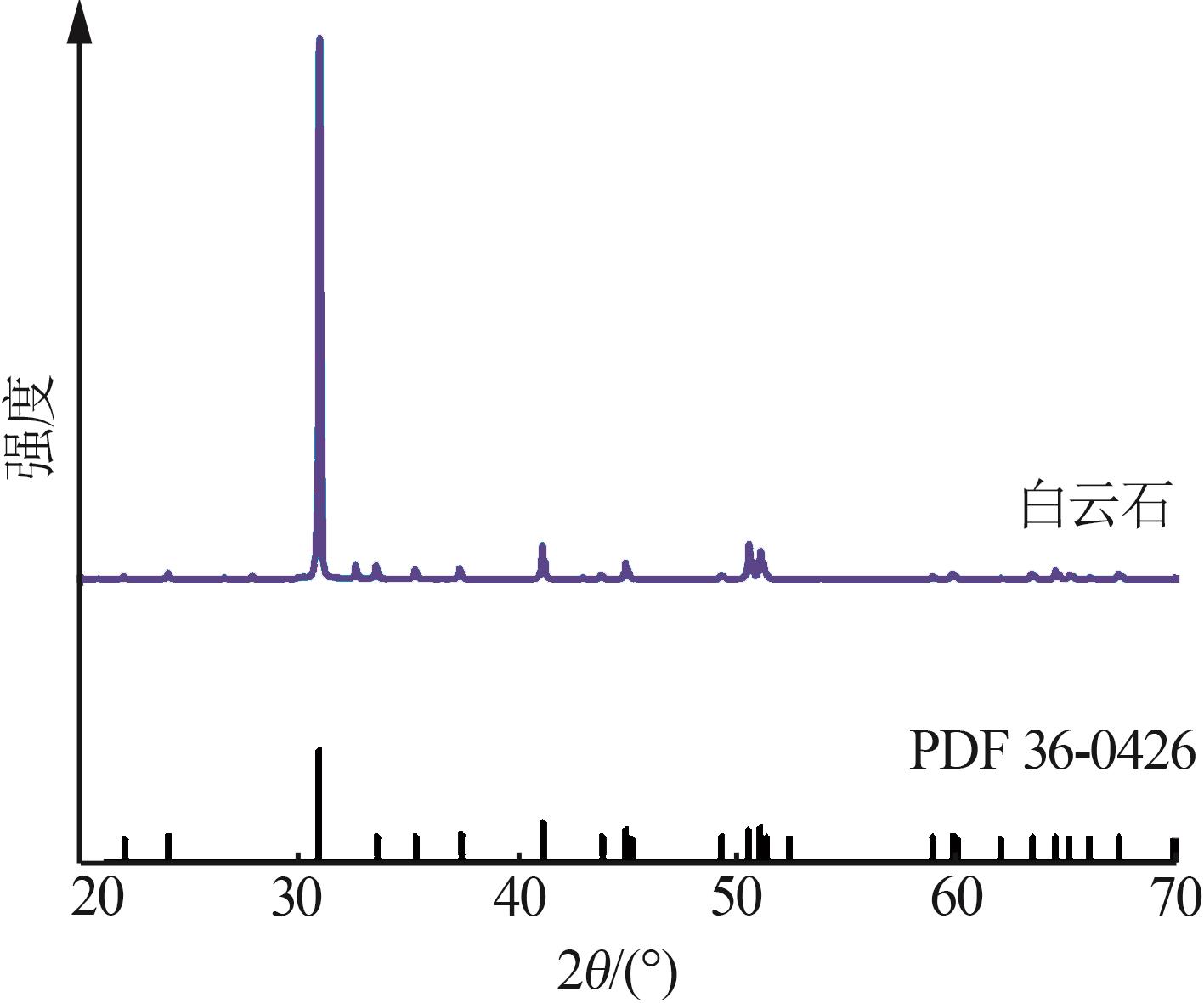
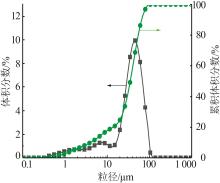
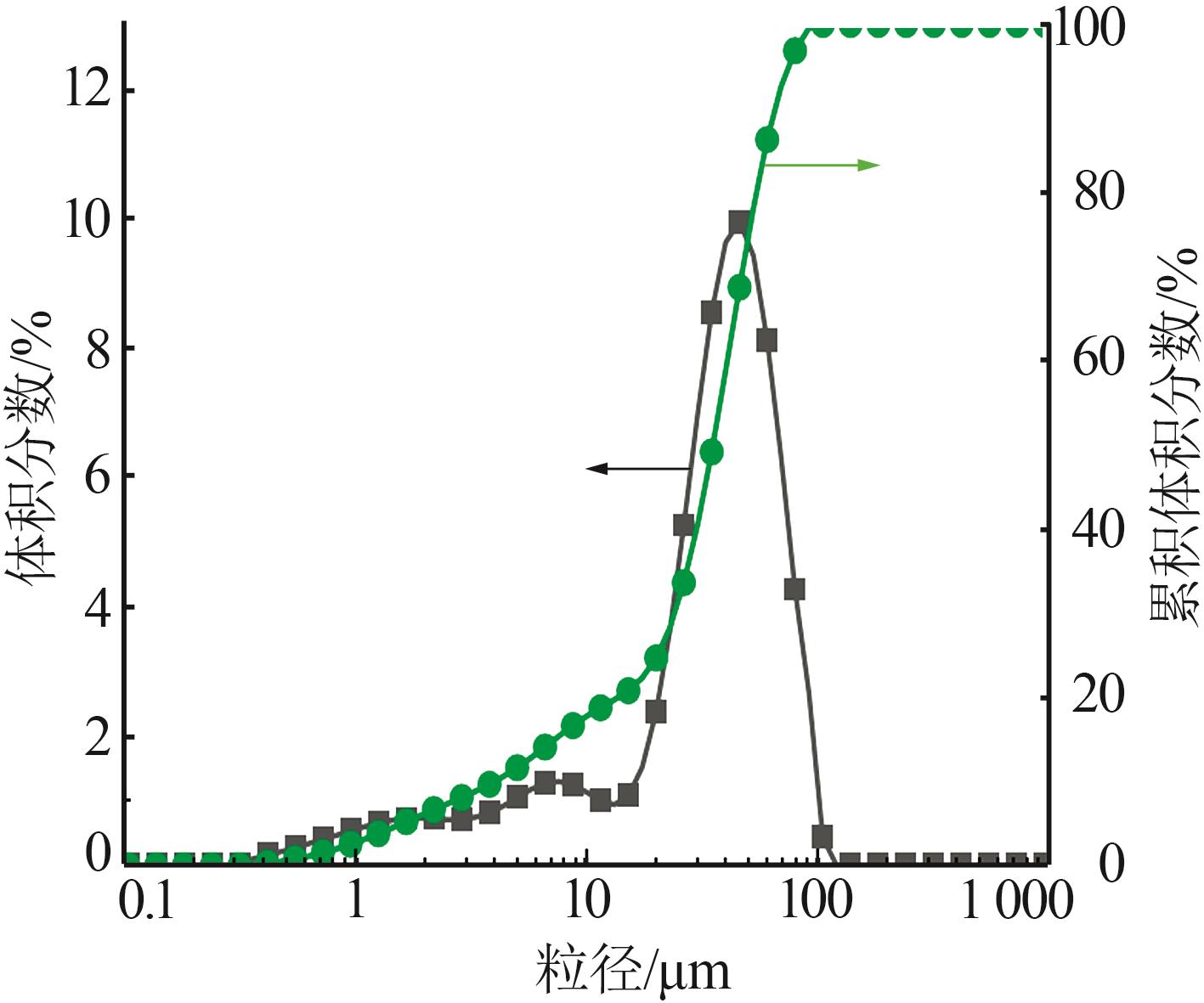
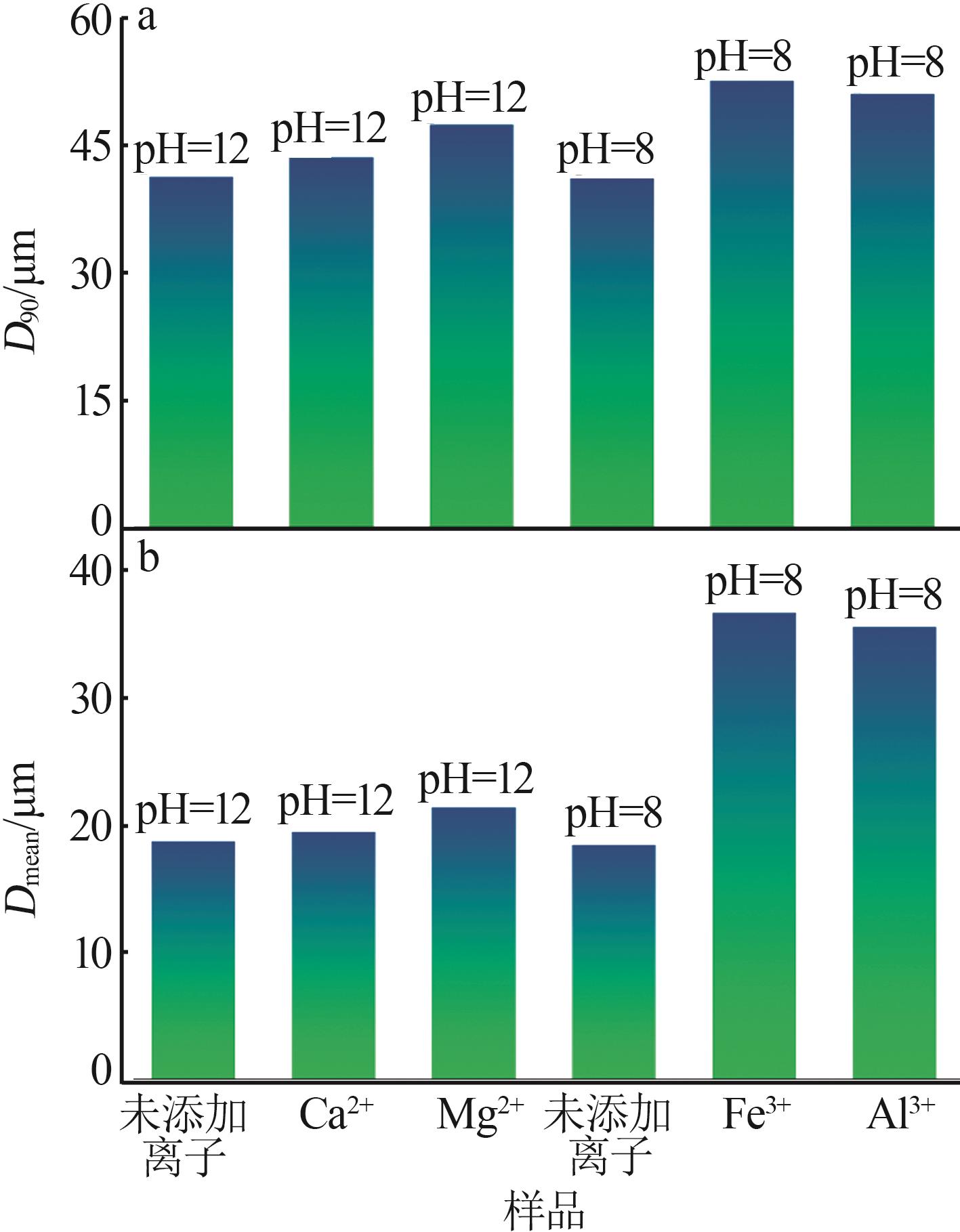
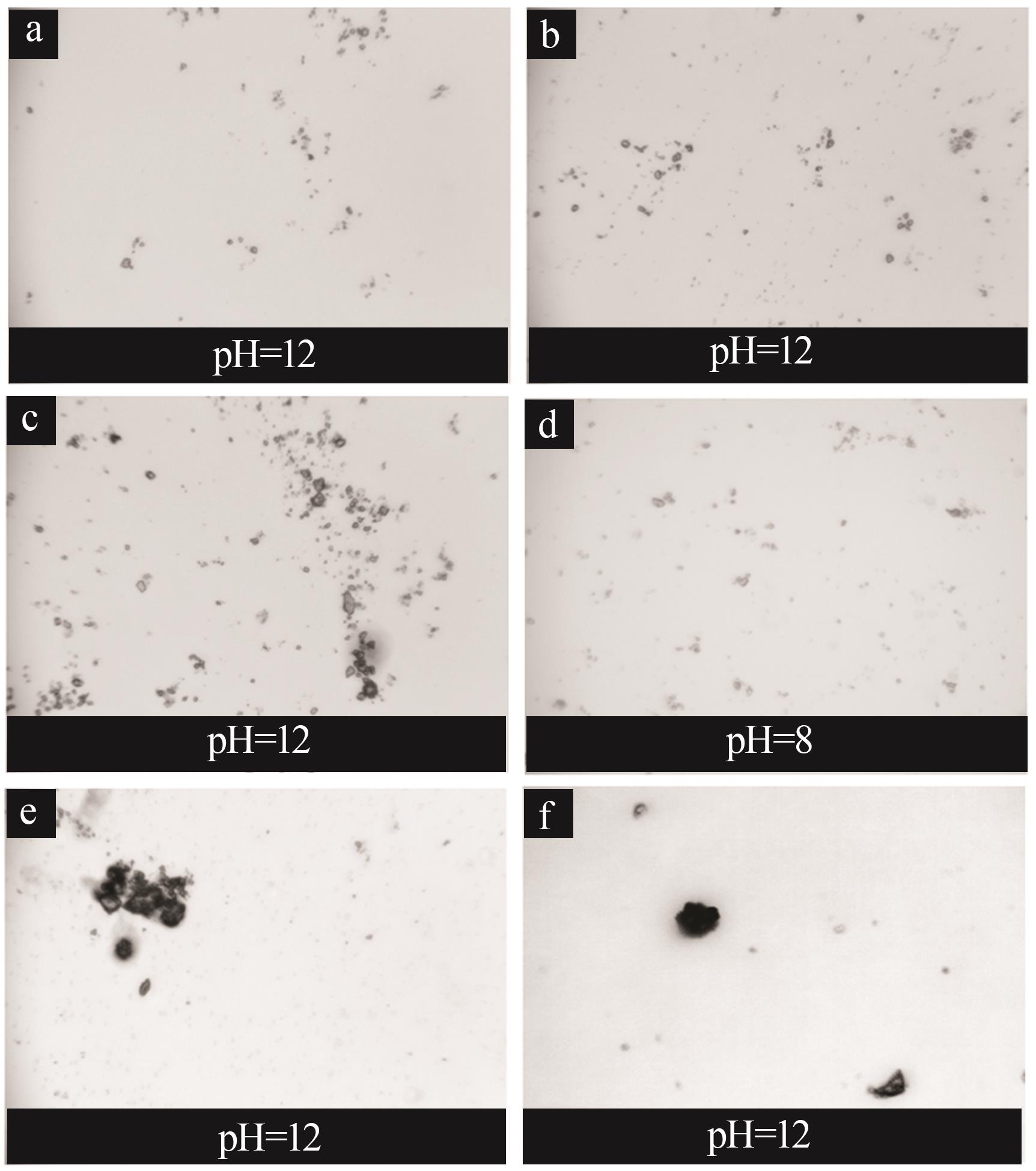
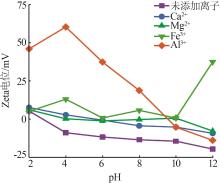
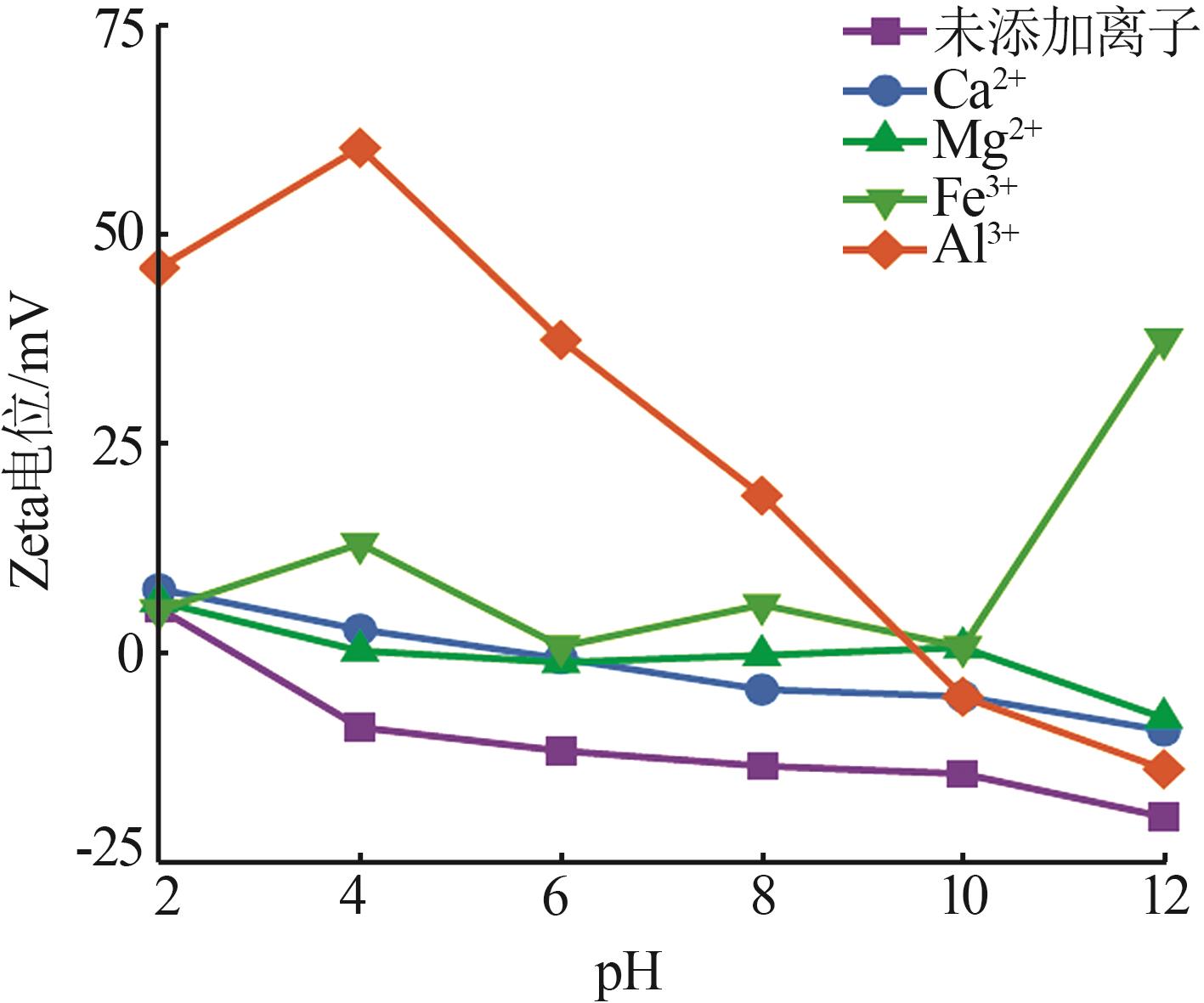
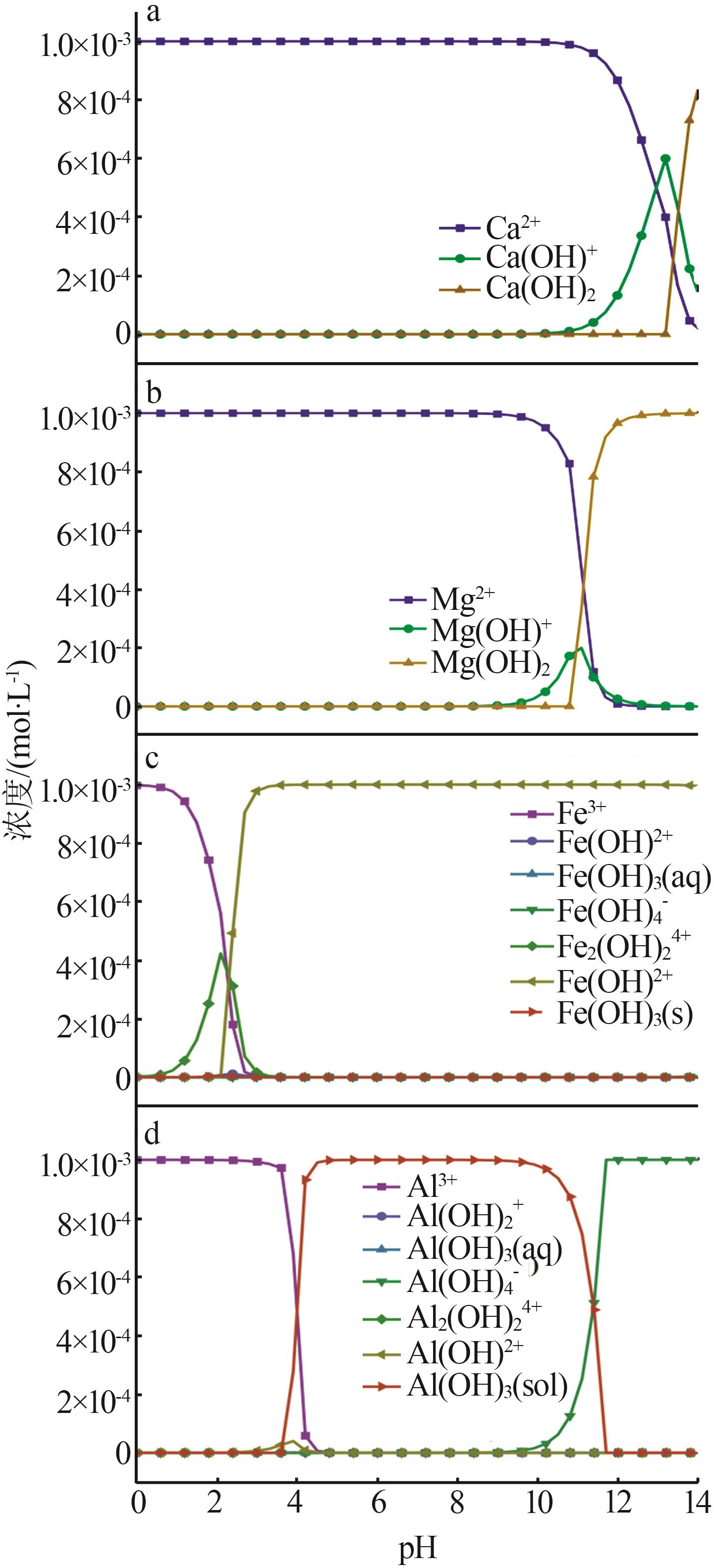
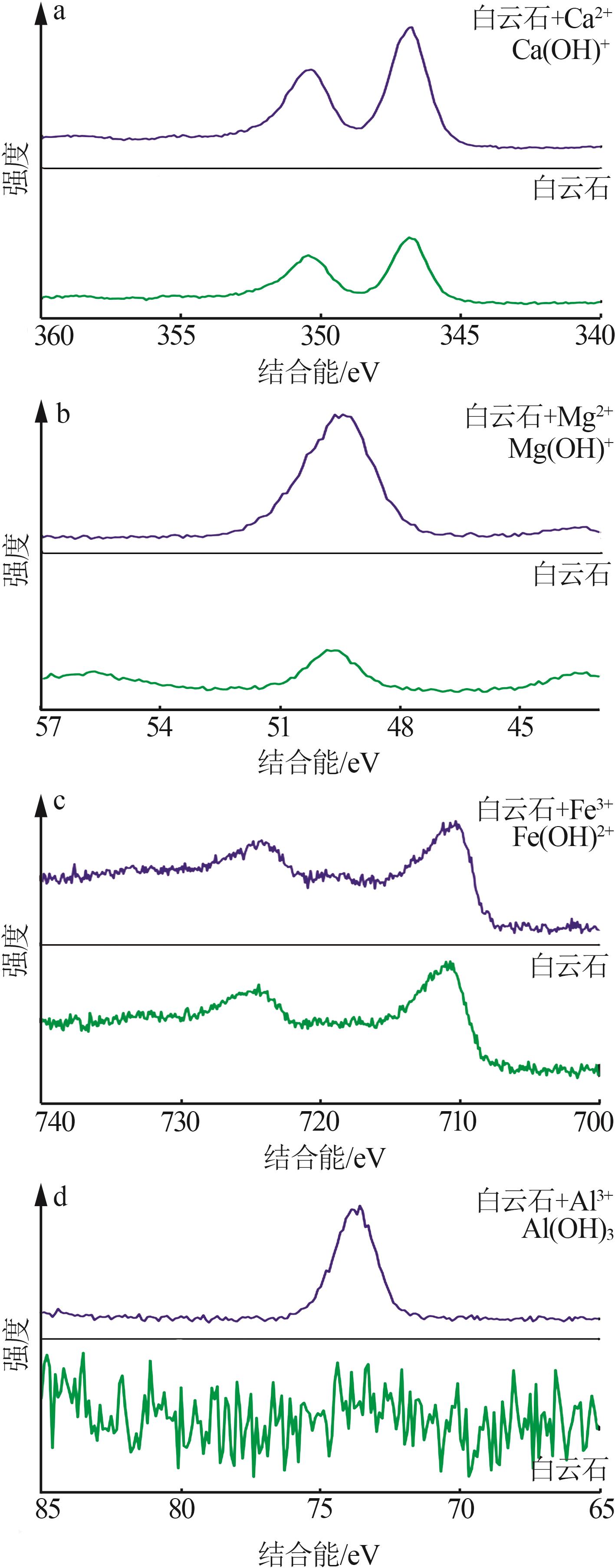
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (2): 44-50.doi: 10.19964/j.issn.1006-4990.2023-0206
• Research & Development • Previous Articles Next Articles
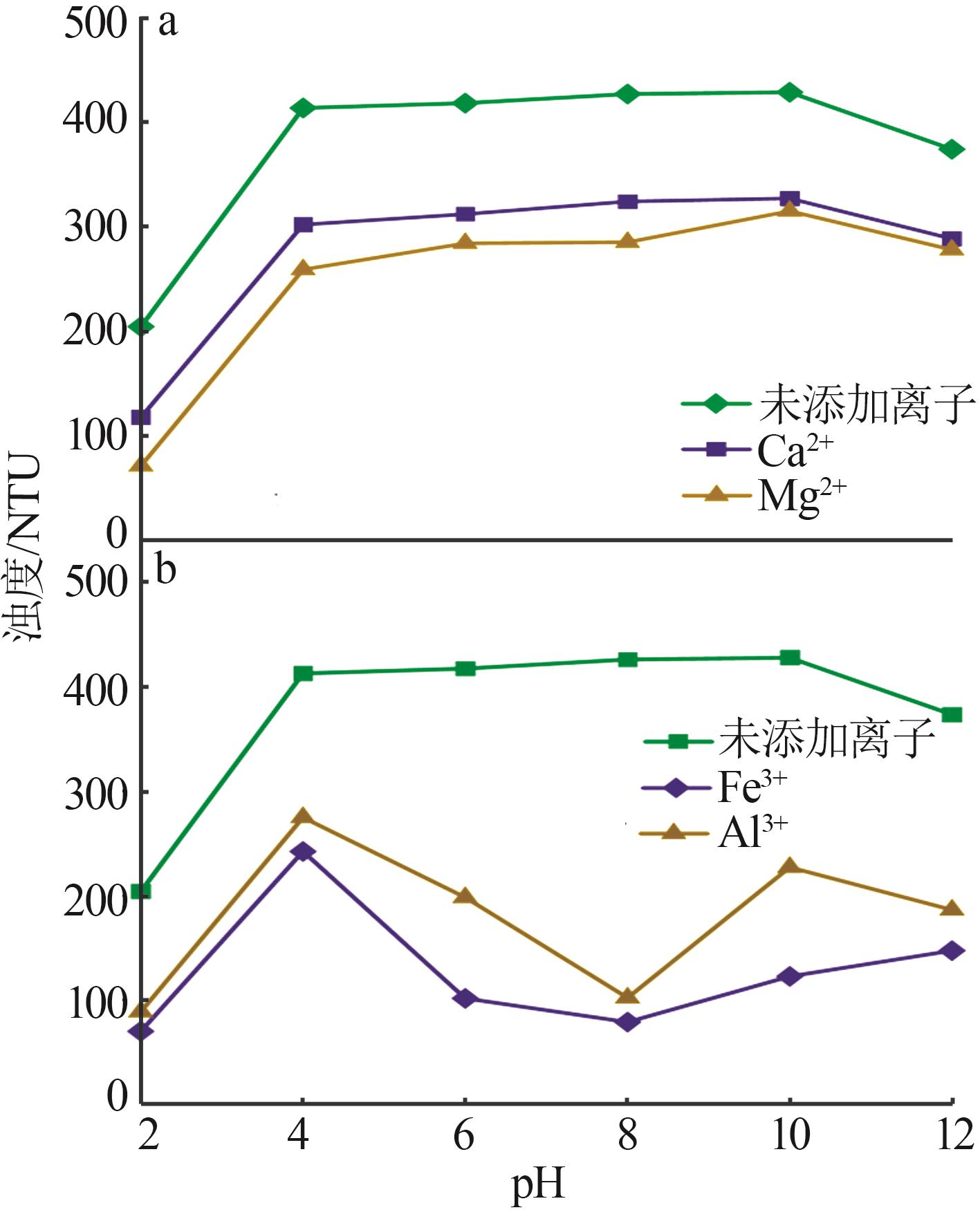
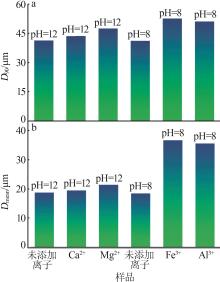
Effect of metallic ions on dispersion behavior of dolomite mineral particles
YUAN Xing( ), DONG Wenyan, WANG Mingshun, CHENG Dilan, AO Xianquan(
), DONG Wenyan, WANG Mingshun, CHENG Dilan, AO Xianquan( )
)
- College of Chemistry and Chemical Engineering,Guizhou University,guiyang 550025,China
-
Received:2023-04-11Online:2024-02-10Published:2024-02-06 -
Contact:AO Xianquan E-mail:3117967934@qq.com;aoxianquan@163.com
CLC Number:
Cite this article
YUAN Xing, DONG Wenyan, WANG Mingshun, CHENG Dilan, AO Xianquan. Effect of metallic ions on dispersion behavior of dolomite mineral particles[J]. Inorganic Chemicals Industry, 2024, 56(2): 44-50.
share this article
| 1 | 韩吉财,朱立新,孙体昌,等.低品位白云石型萤石矿浮选抑制剂作用效果对比研究[J].金属矿山,2019(8):102-107. |
| HAN Jicai, ZHU Lixin, SUN Tichang,et al.Comparative study on the effect of low-grade dolomite-type fluorite ore flotation inhibit-or[J].Metal Mine,2019(8):102-107. | |
| 2 | ZHENG X, SMITH R W.Dolomite depressants in the flotation of apatite and collophane from dolomite[J].Minerals Engineering,1997,10(5):537-545. |
| 3 | BAI Junzhi, WANG Jizhen, YIN Wanzhong,et al.Influence of sodium phosphate salts with different chain length on the flotation behavior of magnesite and dolomite[J].Minerals,2020,10(11):1031. |
| 4 | 张苏江,易锦俊,孔令湖,等.中国磷矿资源现状及磷矿国家级实物地质资料筛选[J].无机盐工业,2016,48(2):1-5,17. |
| ZHANG Sujiang, YI Jinjun, KONG Linghu,et al.Current status of phosphorite-ore resources in China and screening for national-class physical geological data of phopshorite[J].Inorganic Chemicals Industry,2016,48(2):1-5,17. | |
| 5 | MIETTINEN T, RALSTON J, FORNASIERO D.The limits of fine particle flotation[J].Minerals Engineering,2010,23(5):420-437. |
| 6 | SIVAMOHAN R.The problem of recovering very fine particles in mineral processing:A review[J].International Journal of Mineral Processing,1990,28(3/4):247-288. |
| 7 | FARROKHPAY S, FILIPPOV L, FORNASIERO D.Flotation of fine particles:A review[J].Mineral Processing and Extractive Metallurgy Review,2021,42(7):473-483. |
| 8 | MPOFU P, ADDAI-MENSAH J, RALSTON J.Influence of hydrolyzable metal ions on the interfacial chemistry,particle interactions,and dewatering behavior of kaolinite dispersions[J].Journal of Colloid and Interface Science,2003,261(2):349-359. |
| 9 | DIFEO A, FINCH J A, XU Zhenghe.Sphalerite-silica interactions:Effect of pH and calcium ions[J].International Journal of Mineral Processing,2001,61(1):57-71. |
| 10 | MENG Xiangsong, JIANG Min, LIN Shangyong,et al.Metal ions synergize with gangue minerals to remove residual sodium oleate from mineral processing wastewater[J].Journal of Cleaner Production,2023,388:136002. |
| 11 | GAO Zhiyong, JIANG Zheyi, SUN Wei,et al.Typical roles of metal ions in mineral flotation:A review[J].Transactions of Nonferrous Metals Society of China,2021,31(7):2081-2101. |
| 12 | CHEN Junhong, YUAN Xing, YIN Yulan,et al.Effect of Ca2+ dissolution and migration transformation from phosphorite on the surface properties of dolomite[J].Minerals Engineering,2022,188:107824. |
| 13 | CHEN Junhong, AO Xianquan, XIE Yan,et al.Effects of iron ion dissolution and migration from phosphorite on the surface properties of dolomite[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2022,641:128618. |
| 14 | 方启学.钙镁对微细矿粒分散稳定性的影响及其机理研究[J].国外金属矿选矿,1998,35(6):42-45. |
| FANG Qixue.Effect of calcium and magnesium on dispersion stability of fine ore particles and its mechanism[J].Metallic Ore Dressing Abroad,1998,35(6):42-45. | |
| 15 | 王淀佐.浮选溶液化学[M].长沙:湖南科学技术出版社,1988. |
| 16 | 刘忠义.金属离子对菱锌矿和方解石分散行为的影响研究[D].徐州:中国矿业大学,2019. |
| LIU Zhongyi.Study on the influence of metal ions on dispersion behavior of smithsonite and calcite[D].Xuzhou:China University of Mining and Technology,2019. | |
| 17 | LIU R, LIN J, SU W,et al.FTIR and XPS Analysis comparing the activation mechanism pf Ca2+ and Fe3+ on quartz[J].Spectroscopy and Spectral Analysis.2020,40(6):1876-1882 |
| 18 | 刘荣祥,李解,苏文柔,等.红外光谱、 XPS分析比较Ca2+,Fe3+对石英的活化机理[J].光谱学与光谱分析,2020,40(6):1876-1882. |
| LIU Rongxiang, LI Jie, SU Wenrou,et al.FTIR and XPS analysis co-mparing the activation mechanism of Ca2+ and Fe3+ on quartz[J].Spectroscopy and Spectral Analysis,2020,40(6):1876-1882. | |
| 19 | LI Changbin, SHI Qing, ZHANG Guofan,et al.Effect of aluminum ions on the adsorption of carboxymethyl cellulose onto talc[J].Minerals Engineering,2021,170:107018. |
| 20 | 邱冠周,胡岳华,王淀佐.颗粒间相互作用与细粒浮选[M].长沙:中南工业大学出版社,1993. |
| 21 | HARTMANN R, KINNUNEN P, ILLIKAINEN M.Cellulose-mineral interactions based on the DLVO theory and their correlation with flotability[J].Minerals Engineering,2018,122:44-52. |
| 22 | FILBY A, PLASCHKE M, GECKEIS H.AFM force spectroscopy study of carboxylated latex colloids interacting with mineral surfaces[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2012,414:400-414. |
| 23 | YAO Jin.Effects of solution chemistry on the flotation of magnesite,dolomite and quartz with sodium oleate as a collector using SEM analysis[J].Research journal of chemistry and environment.2013,17(7):44-49. |
| [1] | LI Chao, WANG Liping, DAI Yin, GAO Guimei, ZHANG Yunfeng, HONG Yu, XU Lijun, CUI Yongjie. Study on alkali fusion hydrothermal synthesis of 13X zeolite from high silicon tailings and its adsorption on lead,copper and zinc ions [J]. Inorganic Chemicals Industry, 2023, 55(9): 88-93. |
| [2] | QU Xiaoyuan, ZHENG Qiang, FAN Yuanyang, LIU Haili, DENG Xiaoyang, LI Xue. Study on preparation of large cube calcium carbonate by secondary carbonization of dolomite [J]. Inorganic Chemicals Industry, 2023, 55(7): 58-64. |
| [3] | FAN Tianbo,HU Tingting,HAN Dongxue,JIA Xiaohui,ZHAO Yibo,WANG Xin′an,GUO Hongfan,LI Li,LIU Yunyi. Preparation of micro-nano round flake magnesium hydroxide from calcined dolomite powder [J]. Inorganic Chemicals Industry, 2022, 54(1): 29-33. |
| [4] | Fan Tianbo,Jia Xiaohui,Han Dongxue,Chen Si,Jiang Yu,Hu Tingting,Guo Hongfan,Li Li,Liu Yunyi. Study on preparation of vaterite from dolomite by ammonia-alkali method and its mechanism [J]. Inorganic Chemicals Industry, 2021, 53(5): 56-60. |
| [5] | He Liwei,Zhou Xuejin,Ning Ping. Preparation of cement mineral admixture from chromium slag detoxified by carbon monoxide [J]. Inorganic Chemicals Industry, 2021, 53(5): 73-77. |
| [6] | Geng Jing. Study on treatment of arsenic?鄄containing acidic wastewater with lignin?鄄based recapture agent [J]. Inorganic Chemicals Industry, 2019, 51(12): 65-70. |
| [7] | ZHENG Yan, HE Wei-Yan. Study on preparation of magnesium hydroxide with magnesium chloride-dolomite as raw materials [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(8): 52-. |
| [8] | ZHANG Yong-Qiang, SUN Ti-Chang, GUO Qing, HOU Qing-Xia, GUO Ying-Chao, LU Tie-Qiang, YAN Chun-Huan. Research progress on extraction and separation of metal ions with aqueous two-phase system [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(6): 10-. |
| [9] | ZHANG Wang-Nian, DENG Ning, LIANG Wei-Jie, WANG Xin-Hai. Research on digestion process and molding pressure of magnesium calcium sand prepared from dolomite [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(3): 43-. |
| [10] | . Preparation of magnetic ferriferous oxide and its application in purification of heavy metal ions in waste water [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(6): 20-. |
| [11] | CHEN Xia, FENG Wei, JIA Jiu-Chen, ZHU Hua-Bing, QI Yong-Xin, CHEN Li-Fang. Study on carbonation technology of calcined dolomite slurry [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(9): 21-. |
| [12] | GE He-Song, LI Zhou, CAI You-Xing. Study on technological process of calcining and carbonation for production of light magnesium carbonate from dolomite [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(8): 36-. |
| [13] | CHEN Ju-Ling, XIAO Jing-Bo, WANG Wei-Dong. Study on joint-production technology of magnesium compound and potassium sulfate from dolomite [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(7): 35-. |
| [14] | ZHANG Hua, NIE Peng-Fei, XU Chun-He, XU Wang-Sheng. Preparation of high purity magnesium oxide from dolomite [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(4): 22-. |
| [15] | DING Ling- , ZHANG Tian-Lan, GONG Chuang-Zhou, XIA Jun-Ling. Detection of metal ions in potassium hydroxide with inductively coupled plasma optical emission spectrometry method [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(4): 40-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||