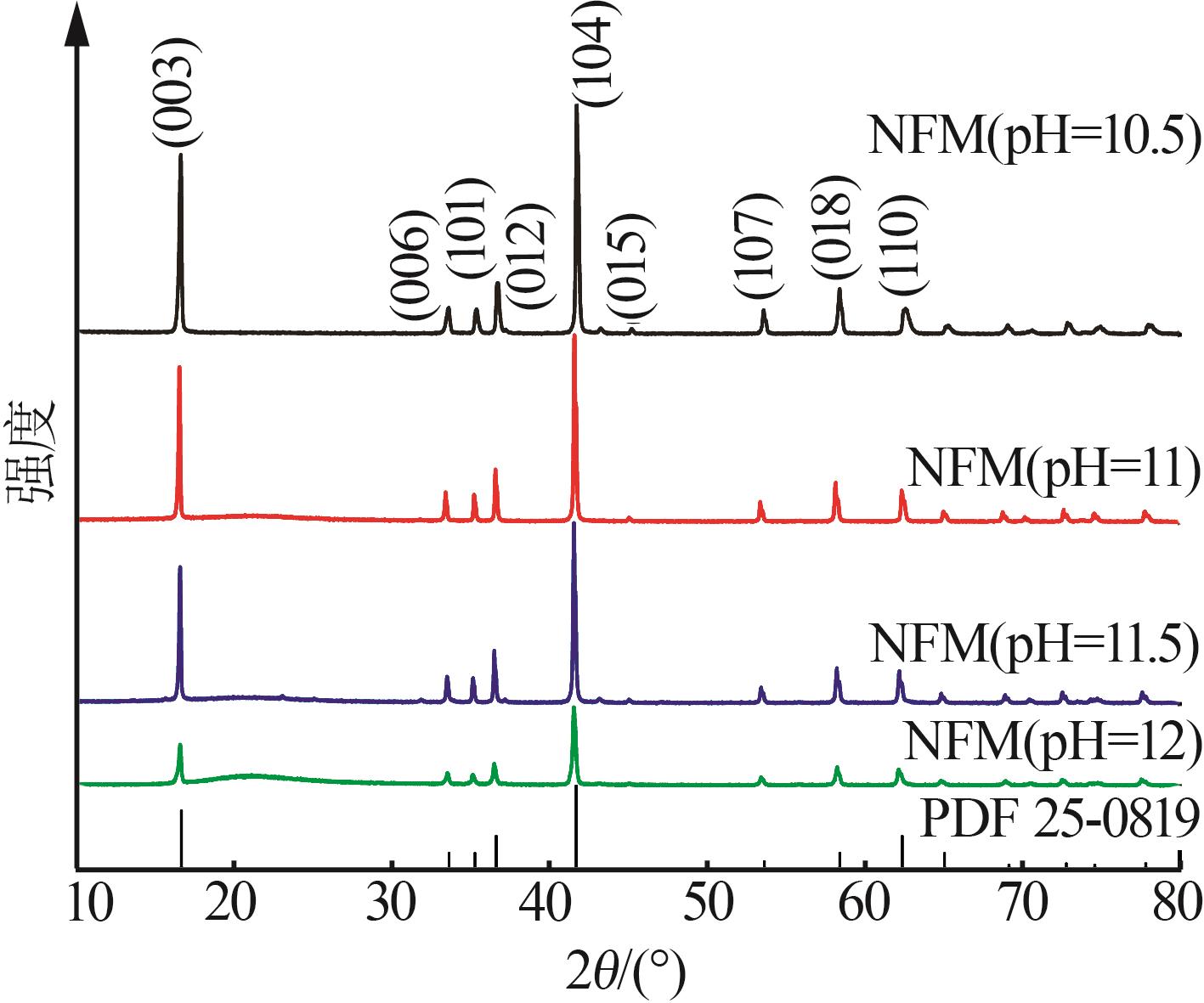
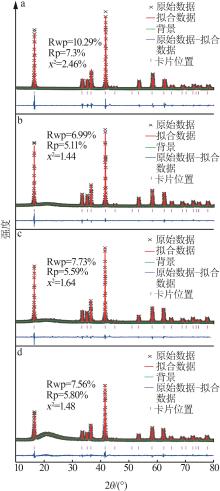
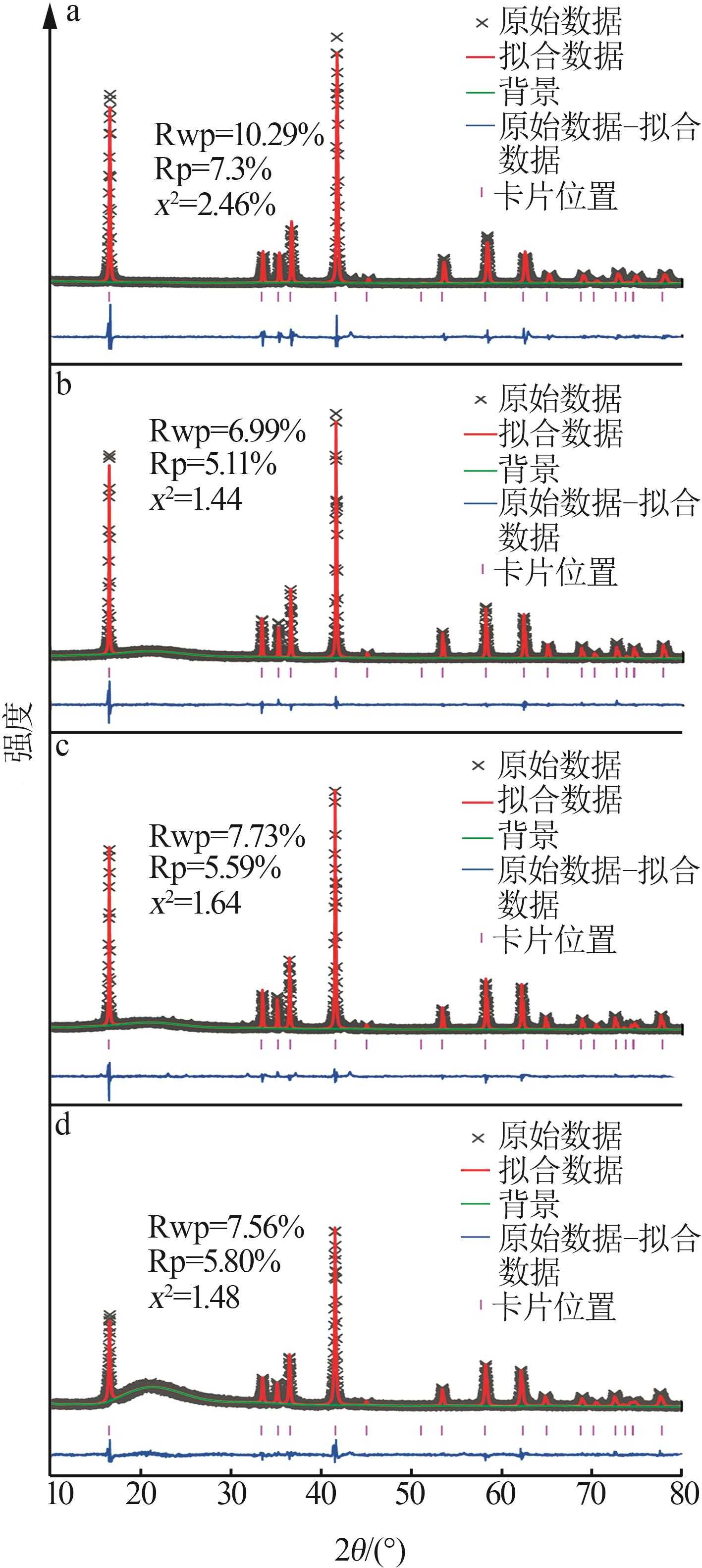
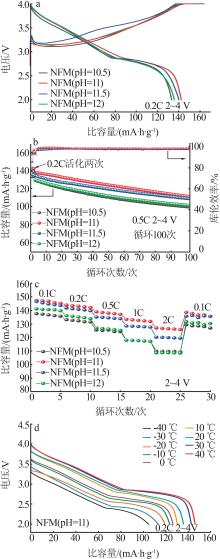
| 1 |
GREY C P, HALL D S.Prospects for lithium-ion batteries and beyond—A 2030 vision[J].Nature Communications,2020,11:6279.
|
| 2 |
YUAN Shuang, LAI Qinghao, DUAN Xiao,et al.Carbon-based materials as anode materials for lithium-ion batteries and lithium-ion capacitors:A review[J].Journal of Energy Storage,2023,61:106716.
|
| 3 |
WANG Yuesheng, XIAO Ruijuan, HU Yongsheng,et al.P2-Na0.6[Cr0.6Ti0.4]O2 cation-disordered electrode for high-rate symmetric rechargeable sodium-ion batteries[J].Nature Communications,2015,6:6954.
|
| 4 |
HWANG J Y, MYUNG S T, SUN Y K.Sodium-ion batteries:Present and future[J].Chemical Society Reviews,2017,46(12):3529-3614.
|
| 5 |
WEI Fanglin, ZHANG Qiaoping, ZHANG Peng,et al.Review—Research progress on layered transition metal oxide cathode materials for sodium ion batteries[J].Journal of the Electrochemical Society,2021,168(5):050524.
|
| 6 |
张亚锋,李宏伟,赵志坚.无人机用锂离子电池正极材料Li1.20Mn0.54Ni0.13Co0.13O2的Mo6+掺杂改性研究[J].无机盐工业,2021,53(11):81-85.
|
|
ZHANG Yafeng, LI Hongwei, ZHAO Zhijian.Study on Mo6+ doping into Li1.20Mn0.54Ni0.13Co0.13O2 as cathode materials for Li-ion batteries applied in unmanned aerial vehicles[J].Inorganic Che-Industry micals,2021,53(11):81-85.
|
| 7 |
HU Hai, TANG Ke, CAO Shuang,et al.Synthesis and electrochemical properties of P2-Na2/3[Ni1/3Mn2/3]O2 microspheres as cathode materials for sodium-ion batteries[J].Journal of Alloys and Compounds,2021,859:157768.
|
| 8 |
张凯,江奥.球形LiMnPO4/C正极材料的喷雾干燥法制备及性能研究[J].无机盐工业,2021,53(1):54-58.
|
|
ZHANG Kai, JIANG Ao.Preparation and properties of spherical LiMnPO4/C cathode materials by spray drying method[J].Inorganic Chemicals Industry,2021,53(1):54-58.
|
| 9 |
XIAO Lifen, JI Fangjie, ZHANG Jiexin,et al.Doping regulation in polyanionic compounds for advanced sodium-ion batteries[J].Small,2023,19(1):e2205732.
|
| 10 |
HE Minglong, DAVIS R, CHARTOUNI D,et al.Assessment of the first commercial Prussian blue based sodium-ion battery[J].Journal of Power Sources,2022,548:232036.
|
| 11 |
WANG Wanlin, GANG Yong, HU Zhe,et al.Reversible structural evolution of sodium-rich rhombohedral Prussian blue for sodium-ion batteries[J].Nature Communications,2020,11:980.
|
| 12 |
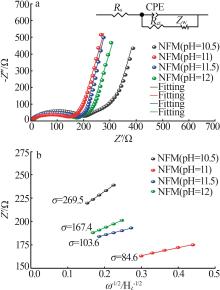
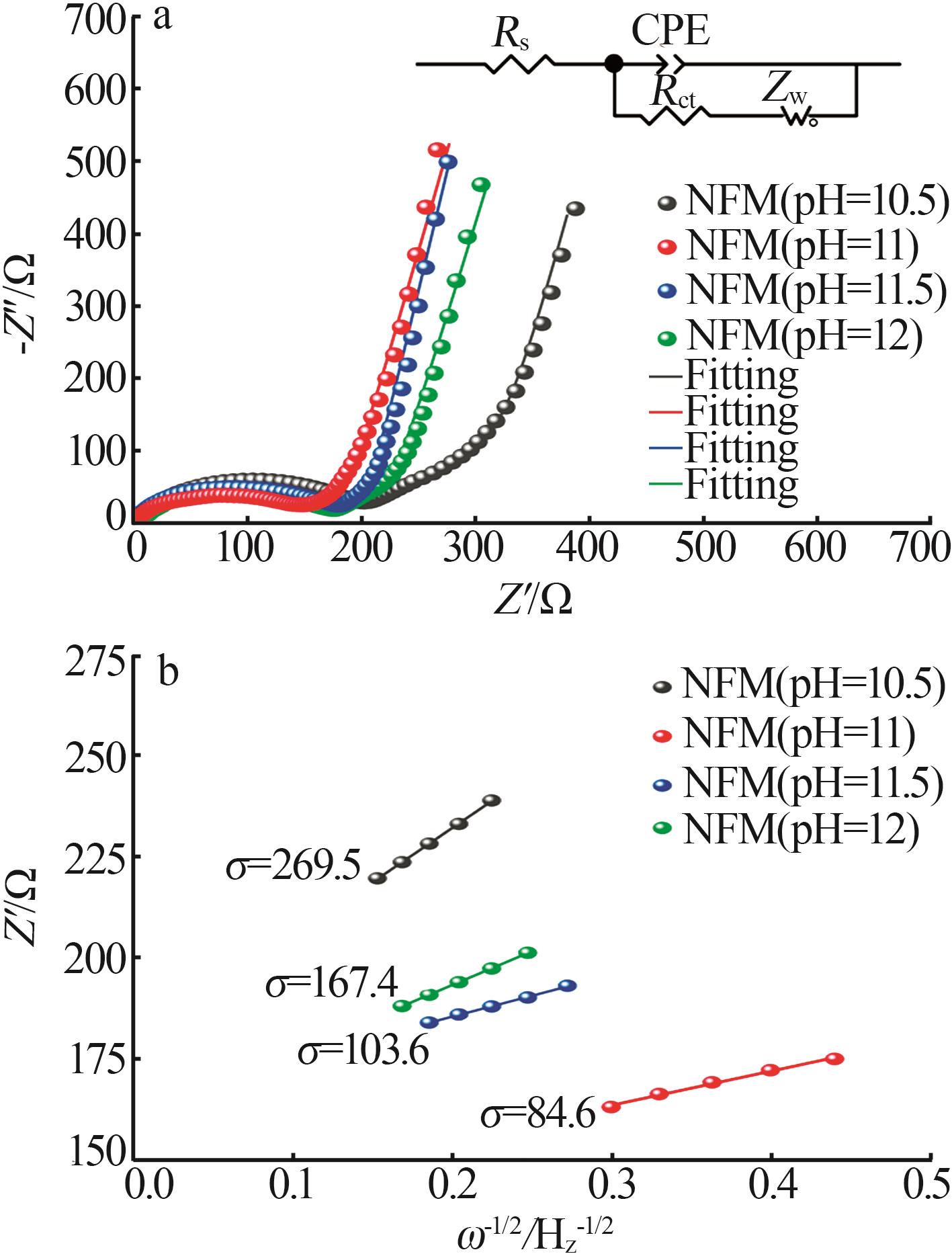
ZUO Daxian, WANG Cuiping, WU Junwei,et al.Effect of co-precipitation pH on the electrochemical properties of Prussian blue electrode materials for sodium-ion batteries[J].Solid State Ionics,2019,336:120-128.
|
| 13 |
DELMAS C, FOUASSIER C, HAGENMULLER P.Structural classification and properties of the layered oxides[J].Physica B+C,1980,99(1/2/3/4):81-85.
|
| 14 |
KIM D, LEE E, SLATER M,et al.Layered Na[Ni1/3Fe1/3Mn1/3]O2 cathodes for Na-ion battery application[J].Electrochemistry Communications,2012,18:66-69.
|
| 15 |
WANG Hong, LIAO Xiaozhen, YANG Yang,et al.Large-scale synthesis of NaNi1/3Fe1/3Mn1/3O2 as high performance cathode materials for sodium ion batteries[J].Journal of the Electrochemical Society,2016,163(3):A565-A570.
|
| 16 |
DING Feixiang, ZHAO Chenglong, ZHOU Dong,et al.A novel Ni-rich O3-Na[Ni0.60Fe0.25Mn0.15]O2 cathode for Na-ion batteries[J].Energy Storage Materials,2020,30:420-430.
|
| 17 |
SUN Yang, WANG Hong, MENG Dechao,et al.Degradation mechanism of O3-type NaNi1/3Fe1/3Mn1/3O2 cathode materials during ambient storage and their in situ regeneration[J].ACS Applied Energy Materials,2021,4(3):2061-2067.
|
| 18 |
SHEN Yabin, WU Yingqiang, XUE Hongjin,et al.Insight into the coprecipitation-controlled crystallization reaction for preparing lithium-layered oxide cathodes[J].ACS Applied Materials & Interfaces,2021,13(1):717-726.
|
| 19 |
XIE Yingying, GAO Han, HARDER R,et al.Revealing the structural evolution and phase transformation of O3-type NaNi1/3Fe1/3Mn1/3O2 cathode material on sintering and cycling processes[J].ACS Applied Energy Materials,2020,3(7):6107-6114.
|
| 20 |
ZHANG S, DENG C, FU B L,et al.Synthetic optimization of spherical Li[Ni1/3Mn1/3Co1/3]O2 prepared by a carbonate co-precipitation method[J].Powder Technology,2010,198(3):373- 380.
|
| 21 |
TOBY B H, VON DREELE R B.GSAS-Ⅱ:The genesis of a modern open-source all purpose crystallography software package[J].Journal of Applied Crystallography,2013,46(2):544-549.
|
| 22 |
TREACY M, NEWSAM J, DEEM M.A general recursion method for calculating diffracted intensities from crystals containing planar faults[J].Proceedings of the Royal Society of London Series A:Mathematical and Physical Sciences,1991,433(1889):499- 520.
|
| 23 |
QIAN Danna.Understanding the surface and interface properties of electrode materials in alkali-ion batteries:A Combination of experimental and computational studies[M].University of California,San Diego,2015.
|
| 24 |
TALYOSEF Y, MARKOVSKY B, LAVI R,et al.Comparing the behavior of nano-and microsized particles of LiMn1.5Ni0.5O4 spinel as cathode materials for Li-ion batteries[J].Journal of the Electrochemical Society,2007,154(7):A682.
|
| 25 |
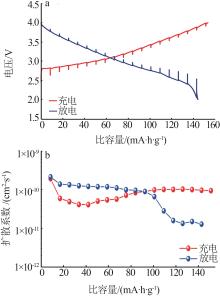
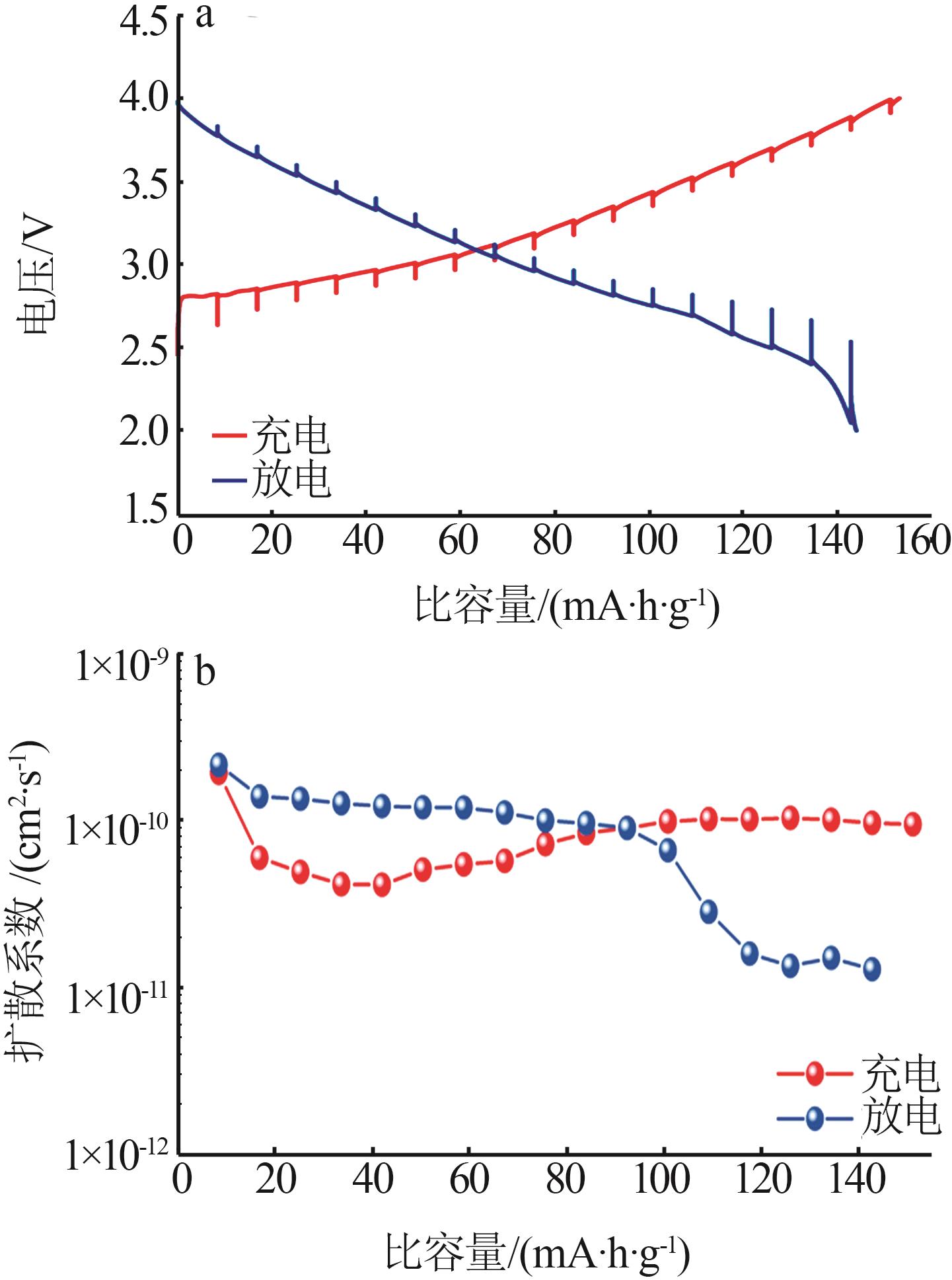
TEO L P, BURAIDAH M H, AROF A K.Study on Li+ ion diffusion in Li2SnO3 anode material by CV and EIS techniques[J].Molecular Crystals and Liquid Crystals,2019,694(1):117-130.
|
| 26 |
DENG Jianqiu, LUO Wenbin, LU Xiao,et al.Sodium-ion batteries:High energy density sodium-ion battery with industrially feasible and air-stable O3-type layered oxide cathode[J].Advanced Energy Materials,2018,8(5):1701610.
|
| 27 |
郭凯强,车海英,张浩然,等.B2O3包覆NaNi1/3Fe1/3Mn1/3O2正极材料制备及其电化学性能[J].储能科学与技术,2022,11(9):2980-2988.
|
|
GUO Kaiqiang, CHE Haiying, ZHANG Haoran,et al.Preparation and characterization of B2O3-coated NaNi1/3Fe1/3Mn1/3O2 cathode materials for sodium-ion batteries[J].Energy Storage Science and Technology,2022,11(9):2980-2988.
|
| 28 |
HOU Ying, JIN Junteng, HUO Chuanrui,et al.New insights into the critical role of inactive element substitution in improving the rate performance of sodium oxide cathode material[J].Energy Storage Materials,2023,56:87-95.
|
 ), HU Xiaomei, WANG Yongxiang, ZHANG Weimin(
), HU Xiaomei, WANG Yongxiang, ZHANG Weimin( )
)