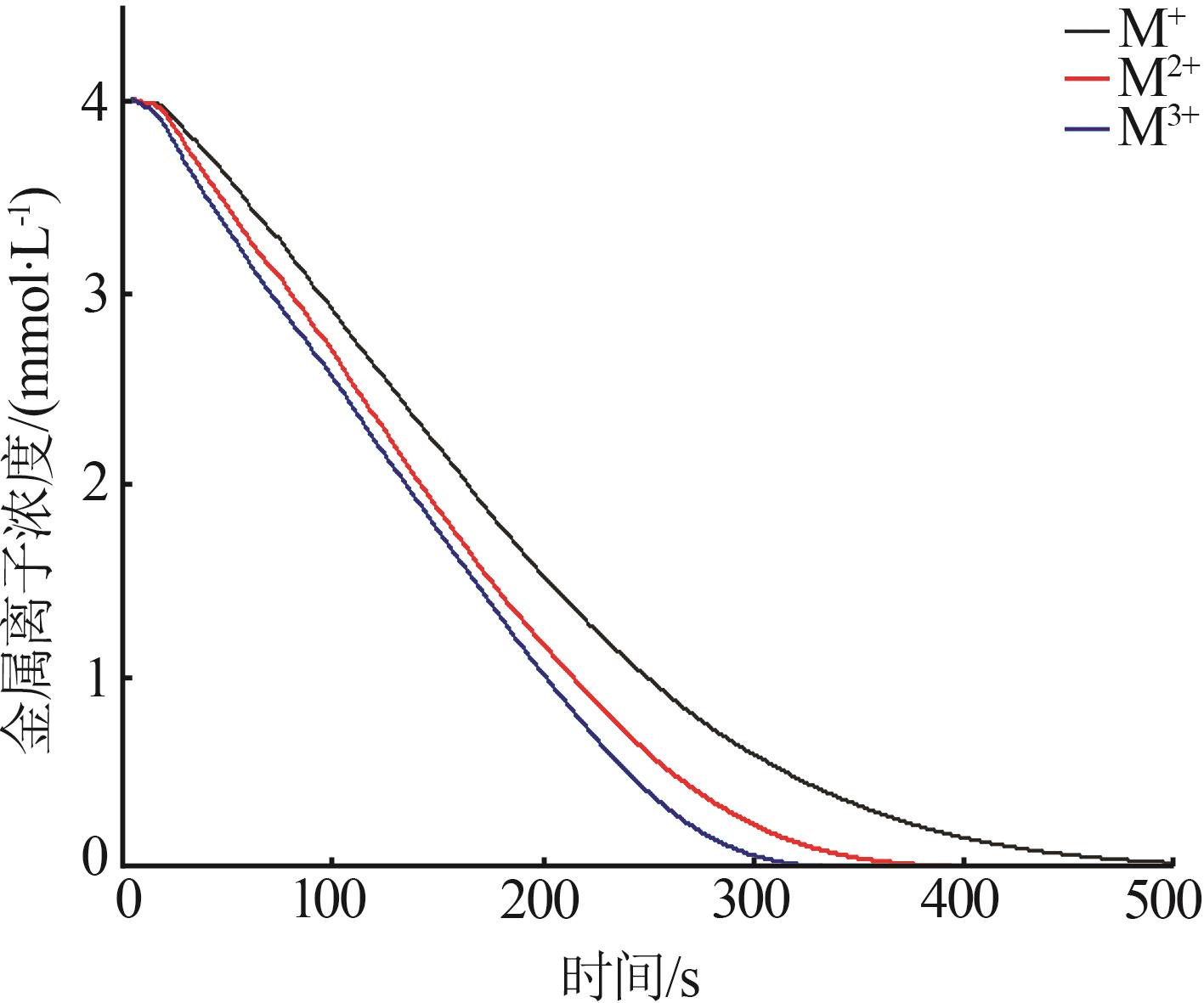
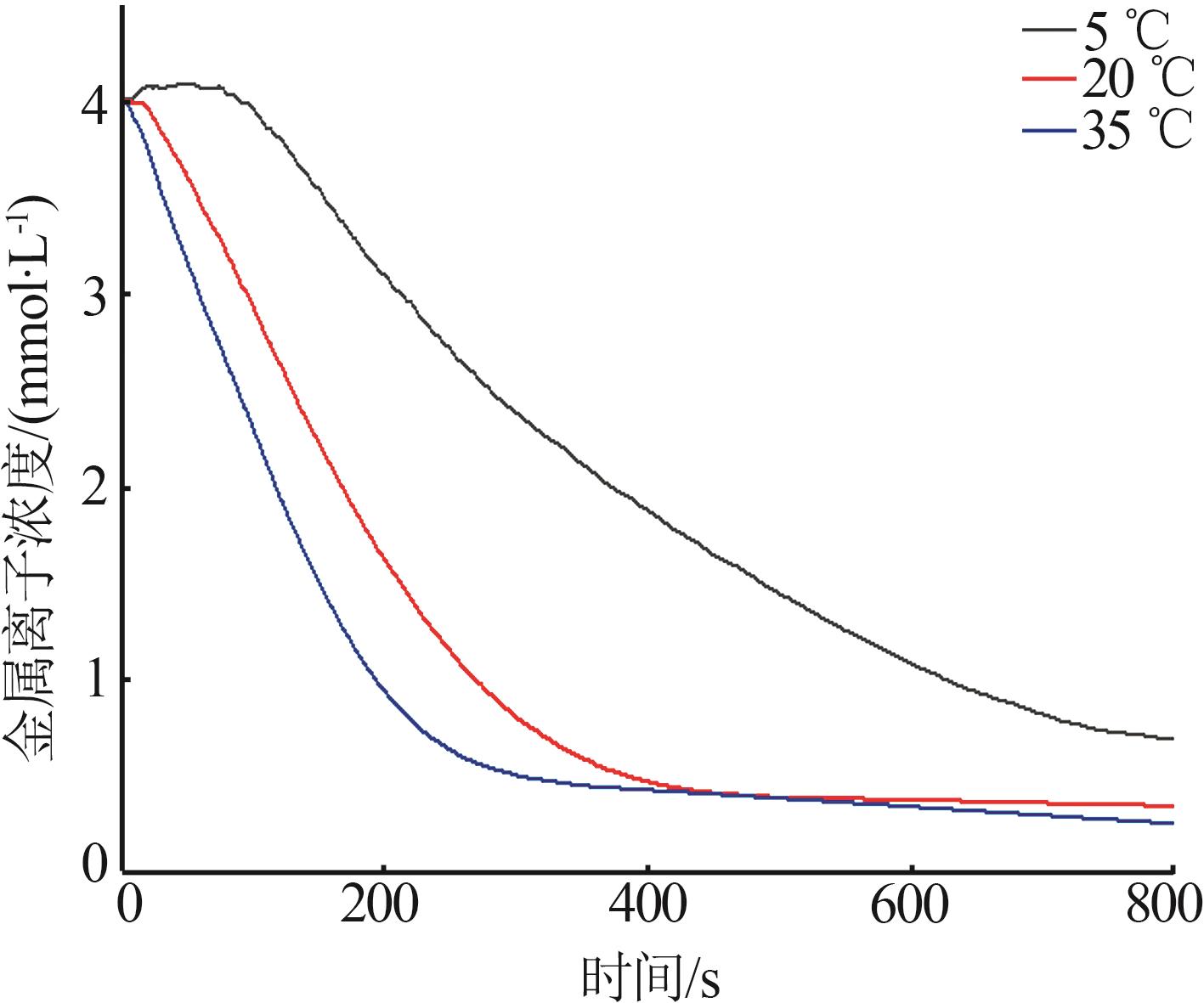
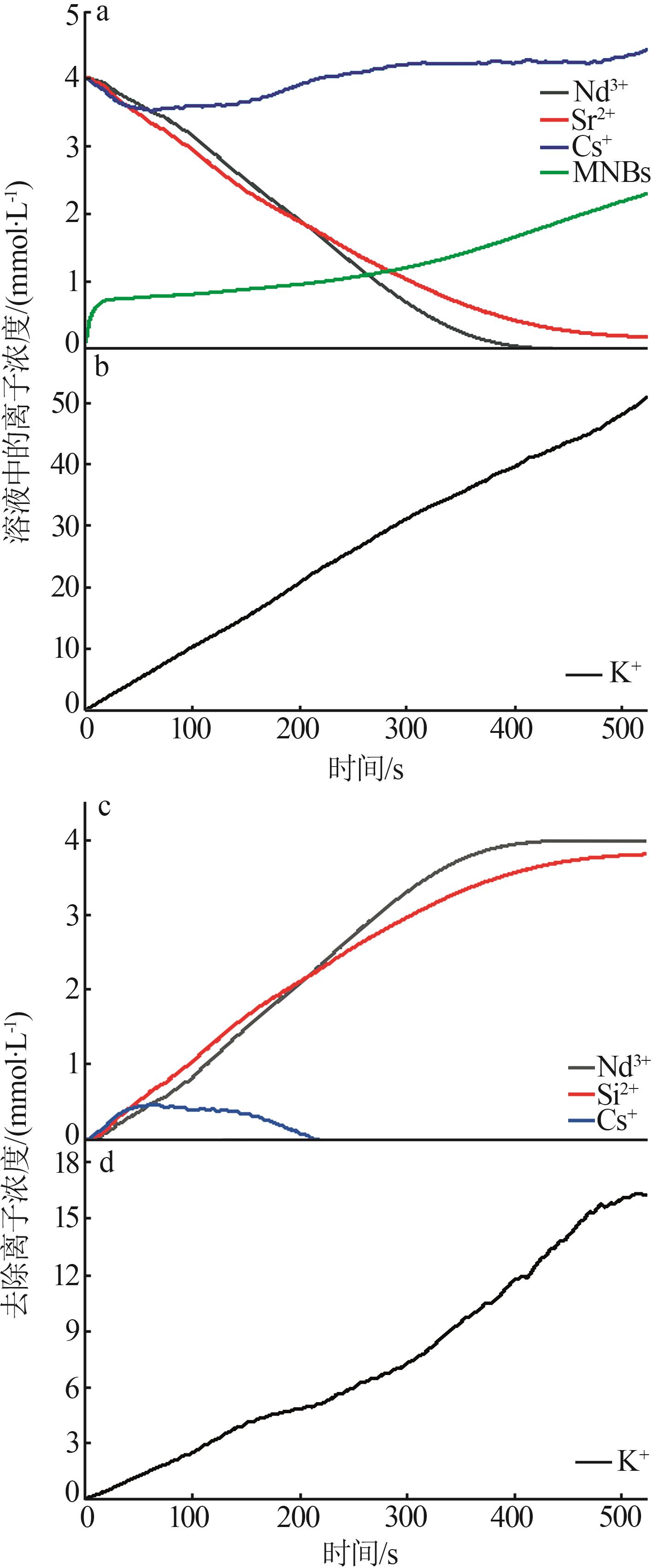
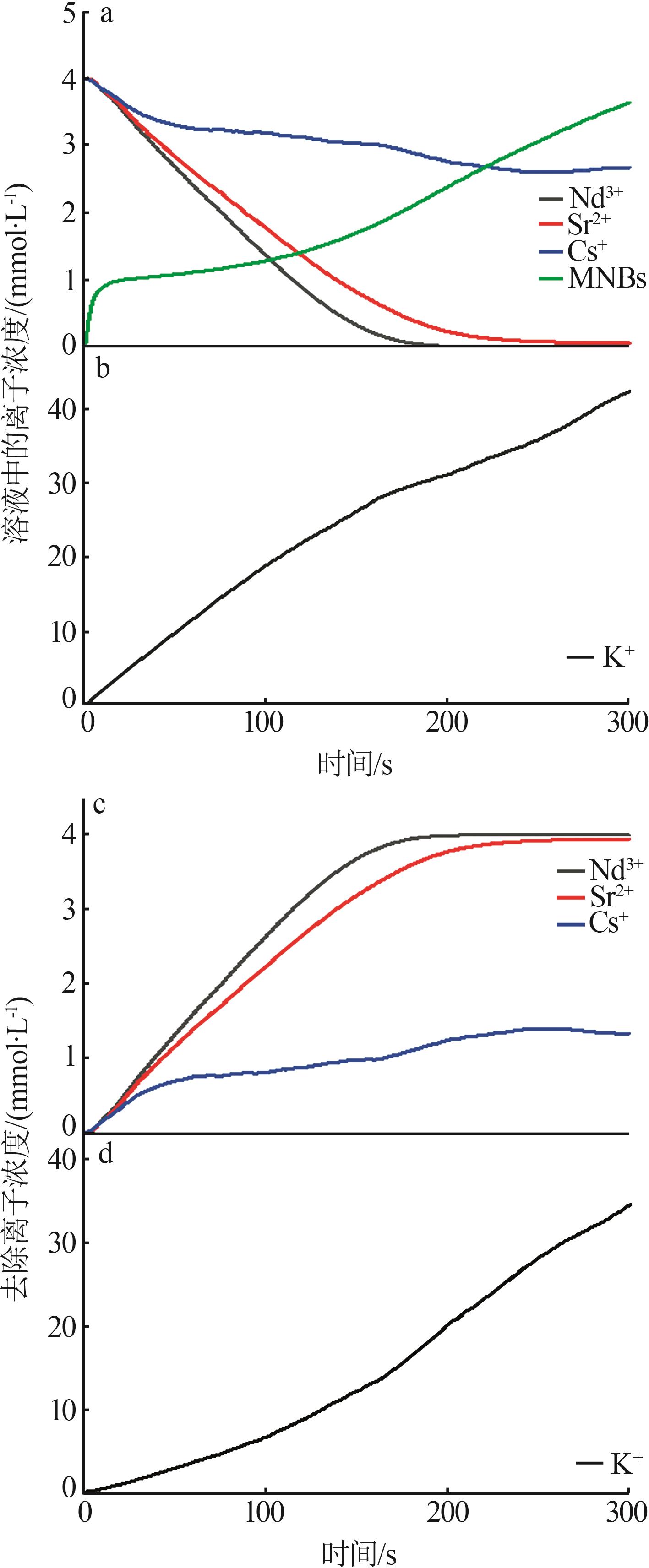
| 1 |
任凤仪,周镇兴.国外核燃料后处理[M].北京:原子能出版社,2006.
|
| 2 |
罗文宗.高放废液中放射性核素分析[J].原子能科学技术,1992,26(5):6-8.
|
|
LUO Wenzong.Determinations of radionuclide in high-level liquid waste(HLLW)[J].Atomic Energy Science and Technology,1992,26(5):6-8.
|
| 3 |
De VICENTE S M G, SMITH N A, El GUEBALY L,et al.Overview on the management of radioactive waste from fusion facilities:ITER,demonstration machines and power plants[J].Nuclear Fusion,2022,62(8):085001.
|
| 4 |
International Atomic Energy Agency.Application of ion exchange processes for the treatment of radioactive waste and management of spent ion exchangers[M].Vienna:International Atomic Energy Agency,2002.
|
| 5 |
LENNEMANN W L.The management of high-level radioactive wastes[J].IAEA Bulletin,1979,21(4):1-16.
|
| 6 |
田立平,鞠玲,王晓波,等.微纳米气泡制备技术及应用研究[J].能源与环境,2020(4):69-73.
|
|
TIAN Liping, JU Ling, WANG Xiaobo,et al. Research on the preparation technology and application of micro and nano bubbl-
|
|
es[J].Energy and Environment,2020(4):69-73.
|
| 7 |
QASEM N A A, MOHAMMED R H, LAWAL D U.Removal of heavy metal ions from wastewater:A comprehensive and critical review[J].Npj Clean Water,2021,4(1):36.
|
| 8 |
TASEIDIFAR M, ZIAEE M, PASHLEY R M,et al.Ion flotation removal of a range of contaminant ions from drinking water[J].Journal of Environmental Chemical Engineering,2019,7(4):103263.
|
| 9 |
XANTHOPOULOS P, BINNEMANS K.Closing the loop in ion flotation:Recovery of copper,regeneration and reuse of collector from the foam phase by a solvometallurgical process[J].Journal of Sustainable Metallurgy,2021,7(4):1565-1574.
|
| 10 |
POOJA G, KUMAR P S, INDRAGANTI S.Recent advancements in the removal/recovery of toxic metals from aquatic system using
|
|
flotation techniques[J].Chemosphere,2022,287:132231.
|
| 11 |
KHATIR M Z, ABDOLLAHY M, KHALESI M R,et al.The selective separation of neodymium from aluminum using ion flotati- on[J].Minerals Engineering,2022,180:107480.
|
| 12 |
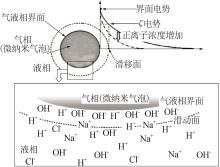
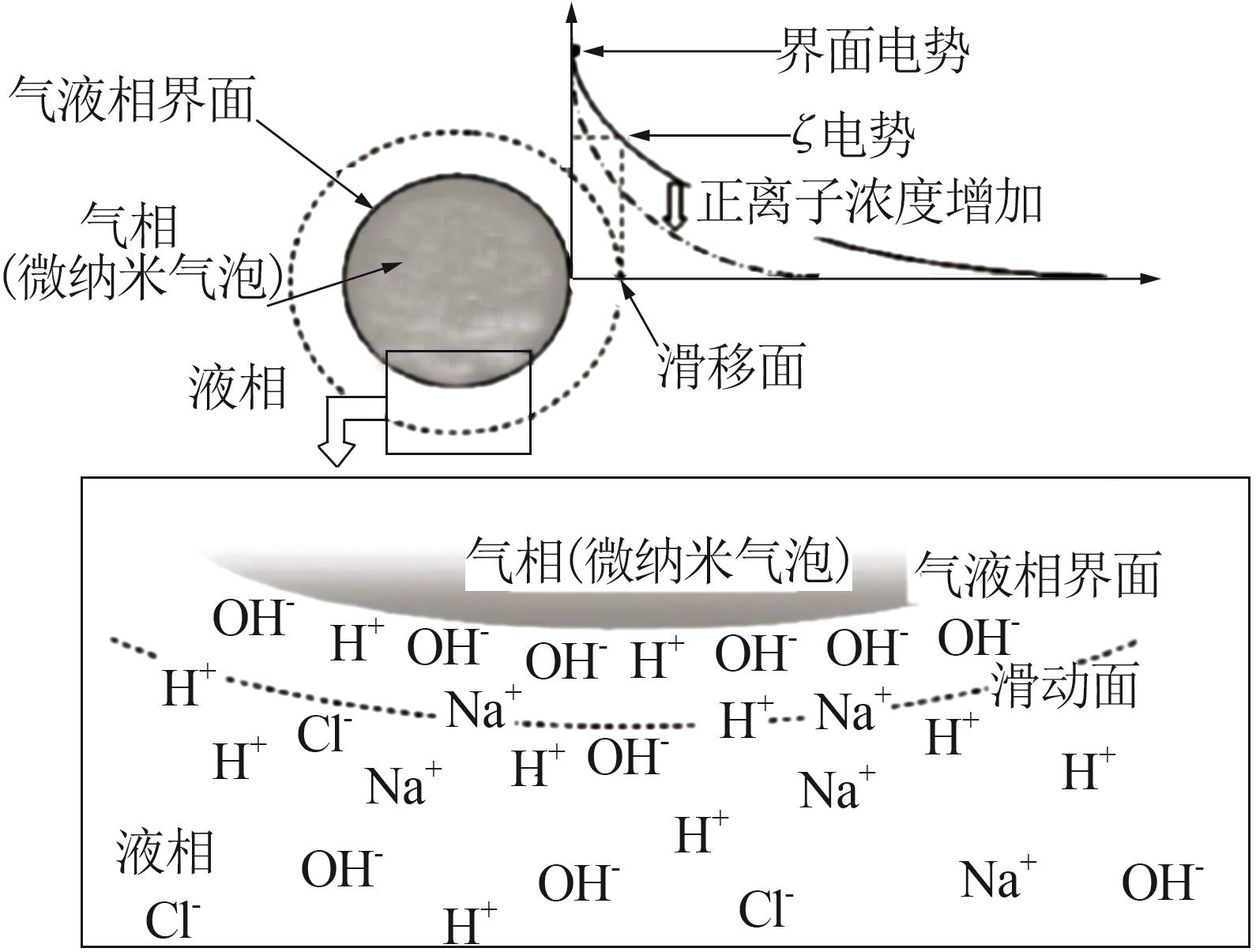
CHO S H, KIM J Y, CHUN J H,et al.Ultrasonic formation of nanobubbles and their zeta-potentials in aqueous electrolyte and surfactant solutions[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2005,269(1/2/3):28-34.
|
| 13 |
TAKAHASHI M. ζ potential of microbubbles in aqueous solutions:Electrical properties of the gas-water interface[J].The Journal of Physical Chemistry B, 2005,109:21858-21864.
|
| 14 |
MEEGODA J N, HEWAGE S A, BATAGODA J H.Application of the diffused double layer theory to nanobubbles[J].Langmuir:the ACS Journal of Surfaces and Colloids,2019,35(37):12100-12112.
|
| 15 |
WILLS B A, FINCH J A.Wills' mineral processing technology[M].8th ed.Boston:Elsevier,2016:265-380.
|
| 16 |
PENG Weijun, CHANG Luping, LI Peiya,et al.An overview on the surfactants used in ion flotation[J].Journal of Molecular Liquids,2019,286:110955.
|
| 17 |
MICHEAU C, SCHNEIDER A, GIRARD L,et al.Evaluation of ion separation coefficients by foam flotation using a carboxylate surfactant[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2015,470:52-59.
|
| 18 |
SMITH R M, MARTELL A E, MOTEKAITIS R J.NIST critically selected stability constants of metal complexes database[Z].NIST standard reference database 46,2004.
|
| 19 |
CHIRKST D E, LOBACHEVA O L, BERLINSKII I V,et al.Recovery and separation of Ce3+ and Y3+ ions from aqueous solutions by ion flotation[J].Russian Journal of Applied Chemistry,2009,82(8):1370-1374.
|
| 20 |
ARSLAN F, BULUT G.Ion flotation and its applications on concentration,recovery,and removal of metal ions from solutions[J].Physicochemical Problems of Mineral Processing,2022,58(5):152061.
|
 ), LI Hongru, SUN Ke, YIN Junlian, ZHOU Wentao(
), LI Hongru, SUN Ke, YIN Junlian, ZHOU Wentao( ), WANG Dezhong
), WANG Dezhong