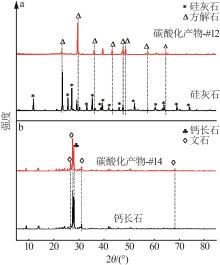
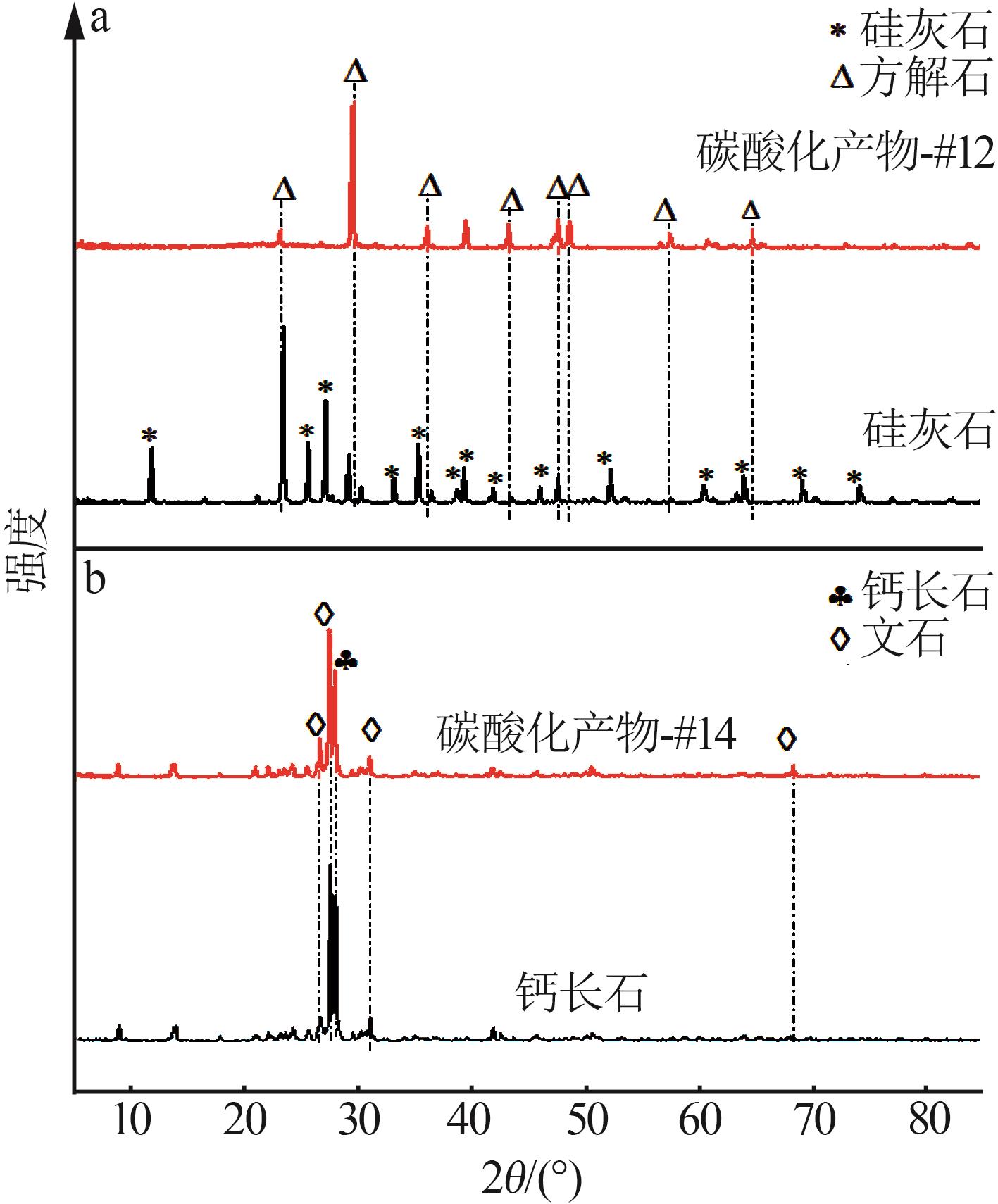
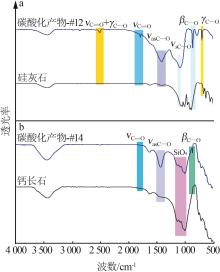
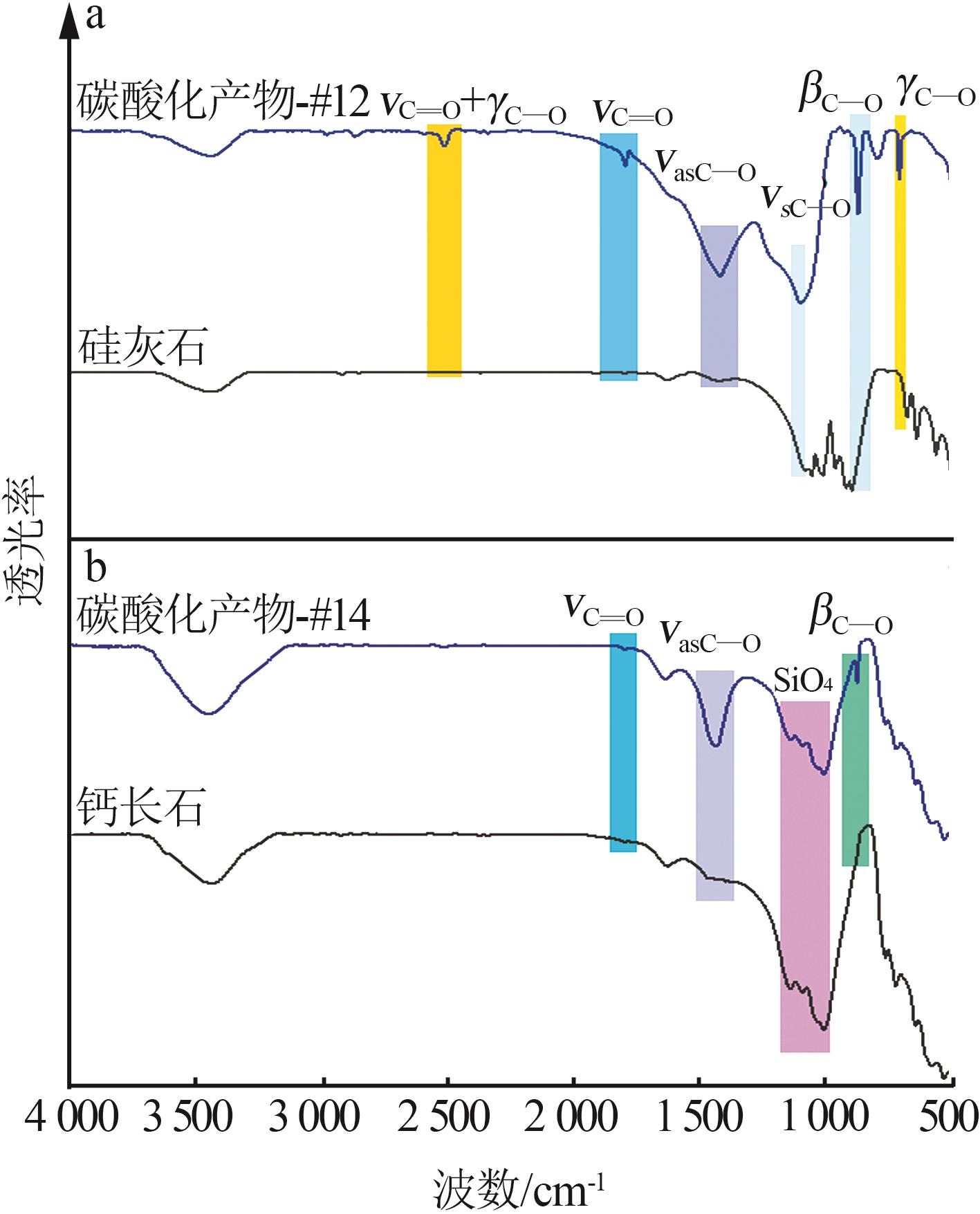
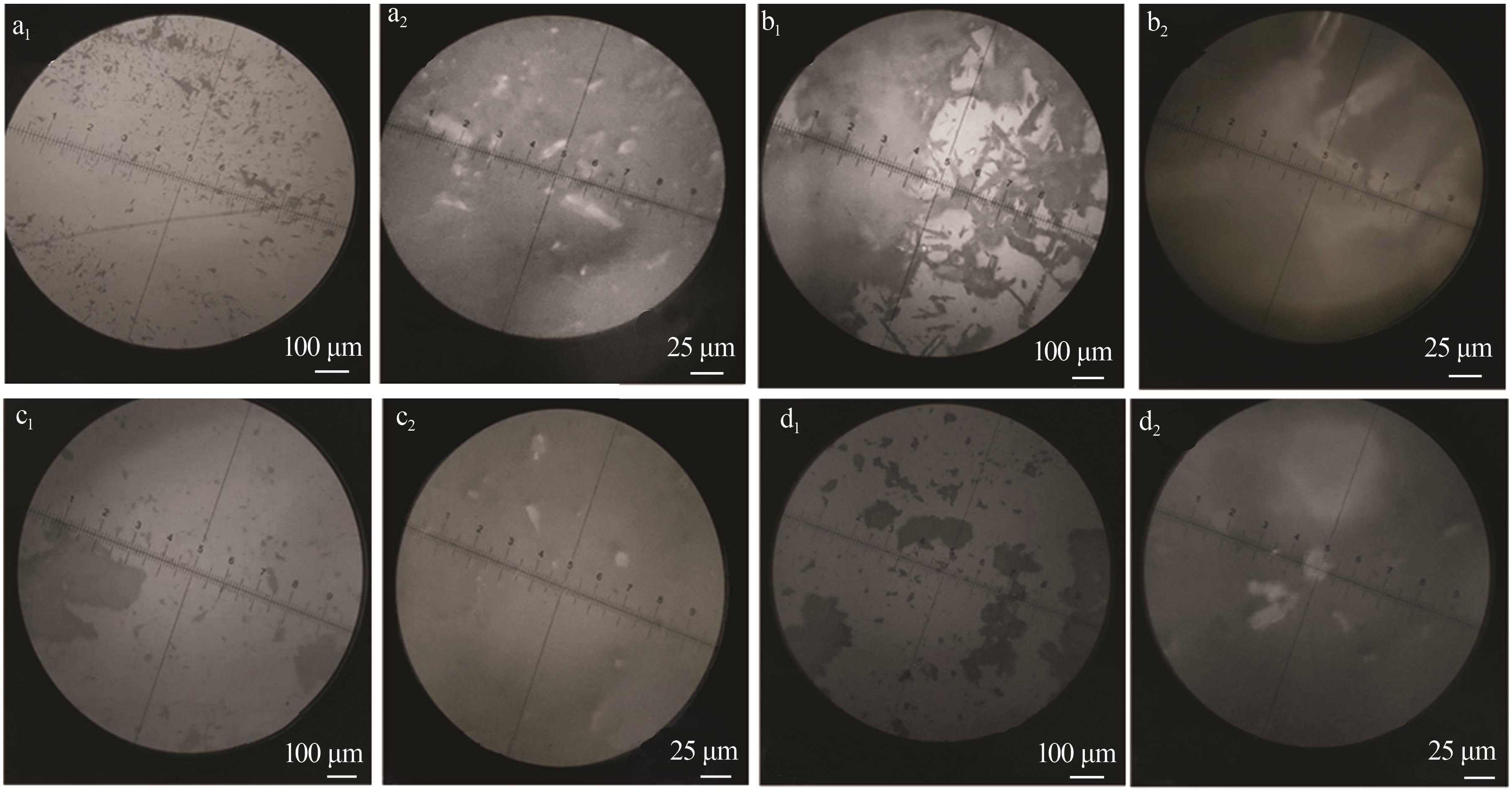
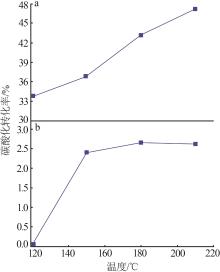
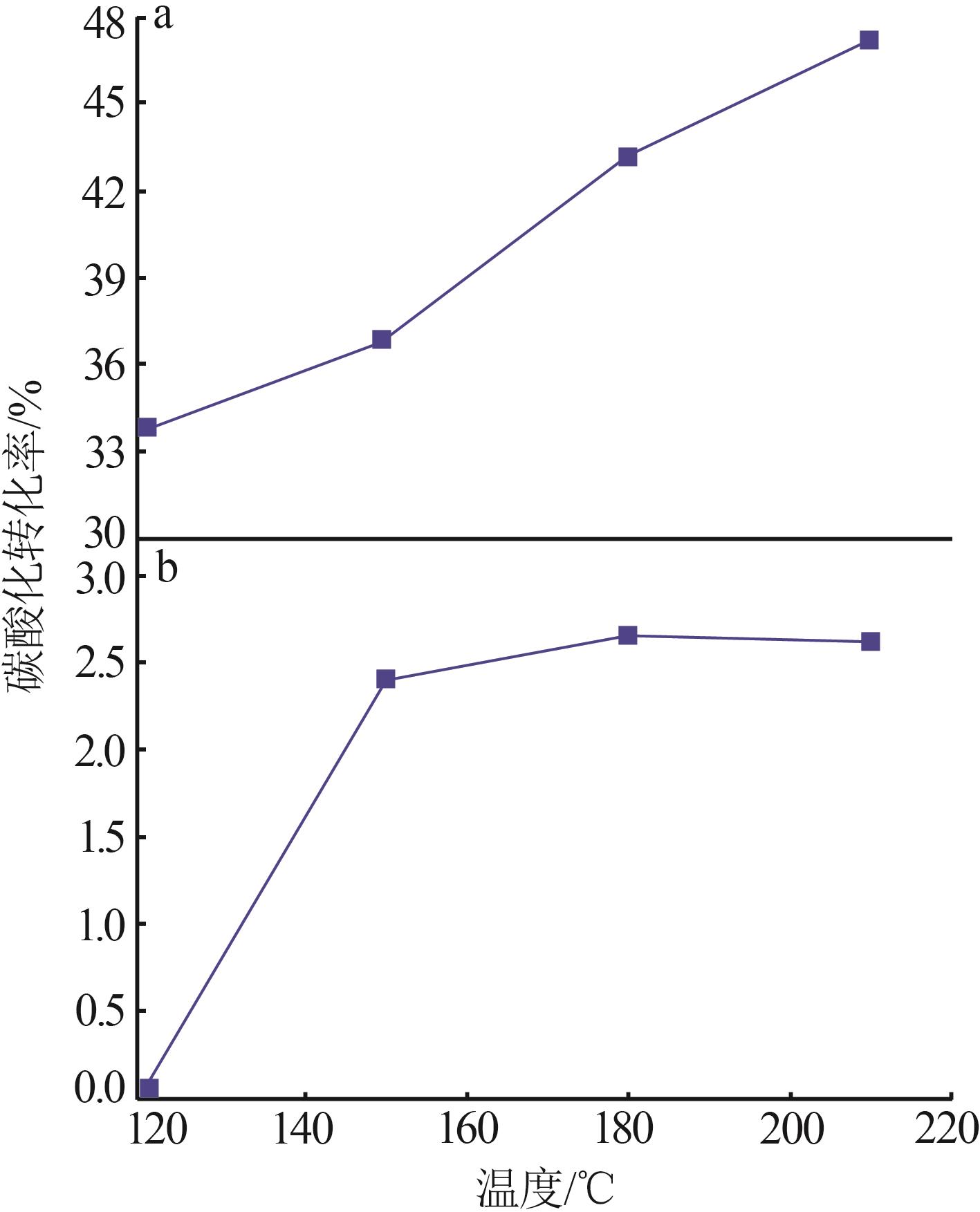
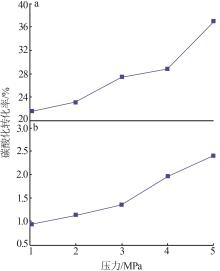
| 1 |
郭伟,石涵,袁标.无机固体吸附剂在二氧化碳捕集应用中的研究进展[J].无机盐工业,2021,53(12):29-34,42.
|
|
GUO Wei, SHI Han, YUAN Biao.Research progress of inorganic solid adsorbents in carbon dioxide capture[J].Inorganic Chemicals Industry,2021,53(12):29-34,42.
|
| 2 |
Yang OU, RONEY C, ALSALAM J,et al.Deep mitigation of CO2 and non-CO2 greenhouse gases toward 1.5 ℃ and 2 ℃ futures[J].Nature Communications,2021,12(1):1-9.
|
| 3 |
JEFFRY L, ONG M Y, NOMANBHAY S,et al.Greenhouse gases utilization:A review[J].Fuel,2021,301:121017.
|
| 4 |
AJAYI T, GOMES J S, BERA A.A review of CO2 storage in geological formations emphasizing modeling,monitoring and capacity estimation approaches[J].Petroleum Science,2019,16(5):1028-1063.
|
| 5 |
何民宇,刘维燥,刘清才,等.CO2矿物封存技术研究进展[J].化工进展,2022,41(4):1825-1833.
|
|
HE Minyu, LIU Weizao, LIU Qingcai,et al.Research progress in CO2 mineral sequestration technology[J].Chemical Industry and Engineering Progress,2022,41(4):1825-1833.
|
| 6 |
蒋剑.钙镁盐直接碳酸化固定CO2实验研究[D].南京:南京师范大学,2018.
|
|
JIANG Jian.Experimental study on CO2 immobilization by direct carbonation of calcium magnesium salt[D].Nanjing:Nanjing Normal University,2018.
|
| 7 |
FAGERLUND J, HIGHFIELD J, ZEVENHOVEN R.Kinetics studies on wet and dry gas-solid carbonation of MgO and Mg(OH)2 for CO2 sequestration[J].RSC Advances,2012,2(27):10380-10393.
|
| 8 |
WANG Bo, PAN Zihe, CHENG Huaigang,et al.A review of carbon dioxide sequestration by mineral carbonation of industrial byproduct gypsum[J].Journal of Cleaner Production,2021,302:126930.
|
| 9 |
RAHMANI O, JUNIN R, TYRER M,et al.Mineral carbonation of red gypsum for CO2 sequestration[J].Energy & Fuels,2014,28(9):5953-5958.
|
| 10 |
李兰兰,叶坤,郭会荣,等.矿物封存二氧化碳实验研究进展[J].资源与产业,2013,15(2):117-123.
|
|
LI Lanlan, YE Kun, GUO Huirong,et al.Test advance in minerals-sealing carbon dioxide[J].Resources & Industries,2013,15(2):117-123.
|
| 11 |
REYNES J F, MERCIER G, BLAIS J F,et al.Feasibility of a mineral carbonation technique using iron-silicate mining waste by direct flue gas CO2 capture and cation complexation using 2,2′-bipyridine[J].Minerals,2021,11(4):343.
|
| 12 |
张兵兵,王慧敏,曾尚红,等.二氧化碳矿物封存技术现状及展望[J].化工进展,2012,31(9):2075-2083.
|
|
ZHANG Bingbing, WANG Huimin, ZENG Shanghong,et al.Current status and outlook of carbon dioxide mineral carbonation technologies[J].Chemical Industry and Engineering Progress,2012,31(9):2075-2083.
|
| 13 |
晏恒.中低压条件下非纯CO2液相直接矿物碳酸化的实验研究[D].武汉:华中科技大学,2015.
|
|
YAN Heng.Experimental study of CO2 sequestration from flue gas by direct on direct aqueous mineral carbonation under low-medium pressure conditions.[D].Wuhan:Huazhong University of Science and Technology,2015.
|
| 14 |
刘瑞,王志华,徐强,等.钙长石矿物的化学活性及其固碳潜力研究初探[J].长春工程学院学报(自然科学版),2013,14(4):64-66,91.
|
|
LIU Rui, WANG Zhihua, XU Qiang,et al.Preliminary studies on anorthite chemical activity and carbon fixation ability[J].Journal of Changchun Institute of Technology(Natural Sciences Edition),2013,14(4):64-66,91.
|
| 15 |
OELKERS E H, GISLASON S R, MATTER J.Mineral carbonation of CO2 [J].Elements,2008,4(5):333-337.
|
| 16 |
EIKELAND E, BLICHFELD A B, TYRSTED C,et al.Optimized carbonation of magnesium silicate mineral for CO2 storage[J].ACS Applied Materials & Interfaces,2015,7(9):5258-5264.
|
| 17 |
张彬,肖霄,韩芸娇,等.碳酸钙三级红外光谱研究[J].无机盐工业,2021,53(1):97-101.
|
|
ZHANG Bin, XIAO Xiao, HAN Yunjiao,et al.Study on third-step infrared spectroscopy of calcium carbonate[J].Inorganic Chemicals Industry,2021,53(1):97-101.
|
| 18 |
KARUNADASA K S P, MANORATNE C H, PITAWALA H M T G A,et al.Thermal decomposition of calcium carbonate(calcite polymorph) as examined by in situ high-temperature X-ray powder diffraction[J].Journal of Physics and Chemistry of Solids,2019,134:21-28.
|
| 19 |
HAN Zuozhen, MENG Ruirui, YAN Huaxiao,et al.Calcium carbonate precipitation by synechocystis sp.PCC6803 at different Mg/Ca molar ratios under the laboratory condition[J].Carbonates and Evaporites,2017,32(4):561-575.
|
| 20 |
纪龙.利用粉煤灰矿化封存二氧化碳的研究[D].北京:中国矿业大学(北京),2018.
|
|
JI Long.Carbon dioxide sequestration by mineralization of coal fly ash[D].Beijing:China University of Mining & Technology,Beijing,2018.
|
| 21 |
LI Jiajie, JACOBS A D, HITCH M.Direct aqueous carbonation on olivine at a CO2 partial pressure of 6.5 MPa[J].Energy,2019,173:902-910.
|
| 22 |
SHENG Haoyi, Li LÜ, LIANG Bin,et al.Aqueous carbonation of the potassium-depleted residue from potassium feldspar-CaCl2 calcination for CO2 fixation[J].Environmental Earth Sciences,2015,73(11):6871-6879.
|
| 23 |
YAN Heng, ZHANG Junying, ZHAO Yongchun,et al.CO2 sequestration by direct aqueous mineral carbonation under low-medium pressure conditions[J].Journal of Chemical Engineering of Japan,2015,48(11):937-946.
|
| 24 |
CHEN Zhongying, O'CONNOR W K, GERDEMANN S J.Chemistry of aqueous mineral carbonation for carbon sequestration and explanation of experimental results[J].Environmental Progress,2006,25(2):161-166.
|
| 25 |
SANNA A, STEEL L, MAROTO-VALER M M.Carbon dioxide sequestration using NaHSO4 and NaOH:A dissolution and carbonation optimisation study[J].Journal of Environmental Management,2017,189:84-97.
|
| 26 |
RUAN Fang, KAWANISHI S, SUKENAGA S,et al.Effect of the silicate structure on calcium elution behaviors of calcium-silicate based mineral phases in aqueous solution[J].ISIJ International,2020,60(3):419-425.
|
| 27 |
王宗华,张军营,徐俊,等.CO2矿物碳酸化隔离的理论研究[J].工程热物理学报,2008,29(6):1063-1068.
|
|
WANG Zonghua, ZHANG Junying, XU Jun,et al.A theoretical study on mineral carbonation for CO2 sequestration[J].Journal of Engineering Thermophysics,2008,29(6):1063-1068.
|
| 28 |
STEWART E M, AGUE J J, FERRY J M,et al.Carbonation and decarbonation reactions:Implications for planetary habitability[J].American Mineralogist,2019,104(10):1369-1380.
|
| 29 |
李亚楠,苏锐,周志超,等.北山新场BS34钻孔岩样在不同温度下的水岩作用[J].核化学与放射化学,2022,44(3):386-392.
|
|
LI Yanan, SU Rui, ZHOU Zhichao,et al.Water-rock interaction of granite from borehole BS34 at different temperatures[J].Journal of Nuclear and Radiochemistry,2022,44(3):386-392.
|
| 30 |
SORAI M, SASAKI M.Dissolution kinetics of anorthite in a supercritical CO2-water system[J].American Mineralogist,2010,95(5/6):853-862.
|
| 31 |
姚曦智.超临界二氧化碳环境下钙镁矿物碳酸盐化实验研究[D].南京:南京大学,2017.
|
|
YAO Xizhi.Study of calcium and magnesium mineral carbonation under supercritical CO2 condition[D].Nanjing:Nanjing University,2017.
|
 ), LIU Shengyu1,2,3(
), LIU Shengyu1,2,3( ), ZHANG Lei1, YANG Zhichao1,2, GUO Jianying1,2, LI Bao1,2
), ZHANG Lei1, YANG Zhichao1,2, GUO Jianying1,2, LI Bao1,2