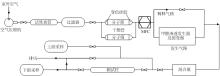
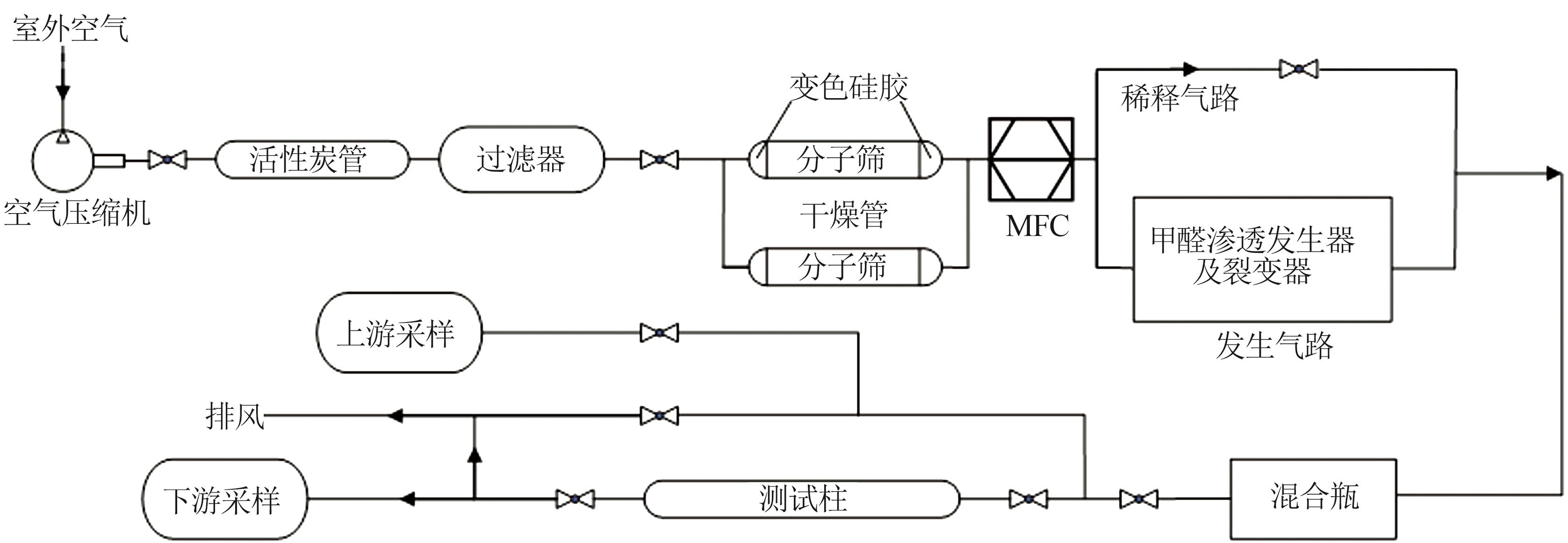
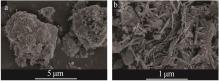
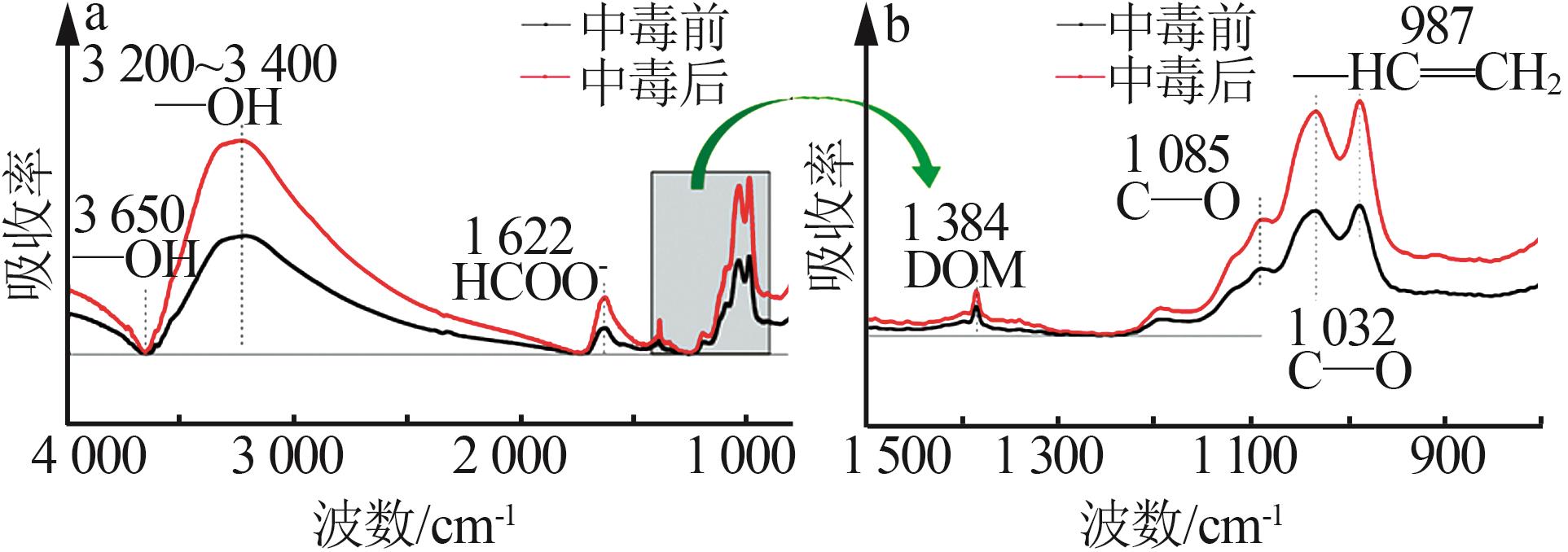
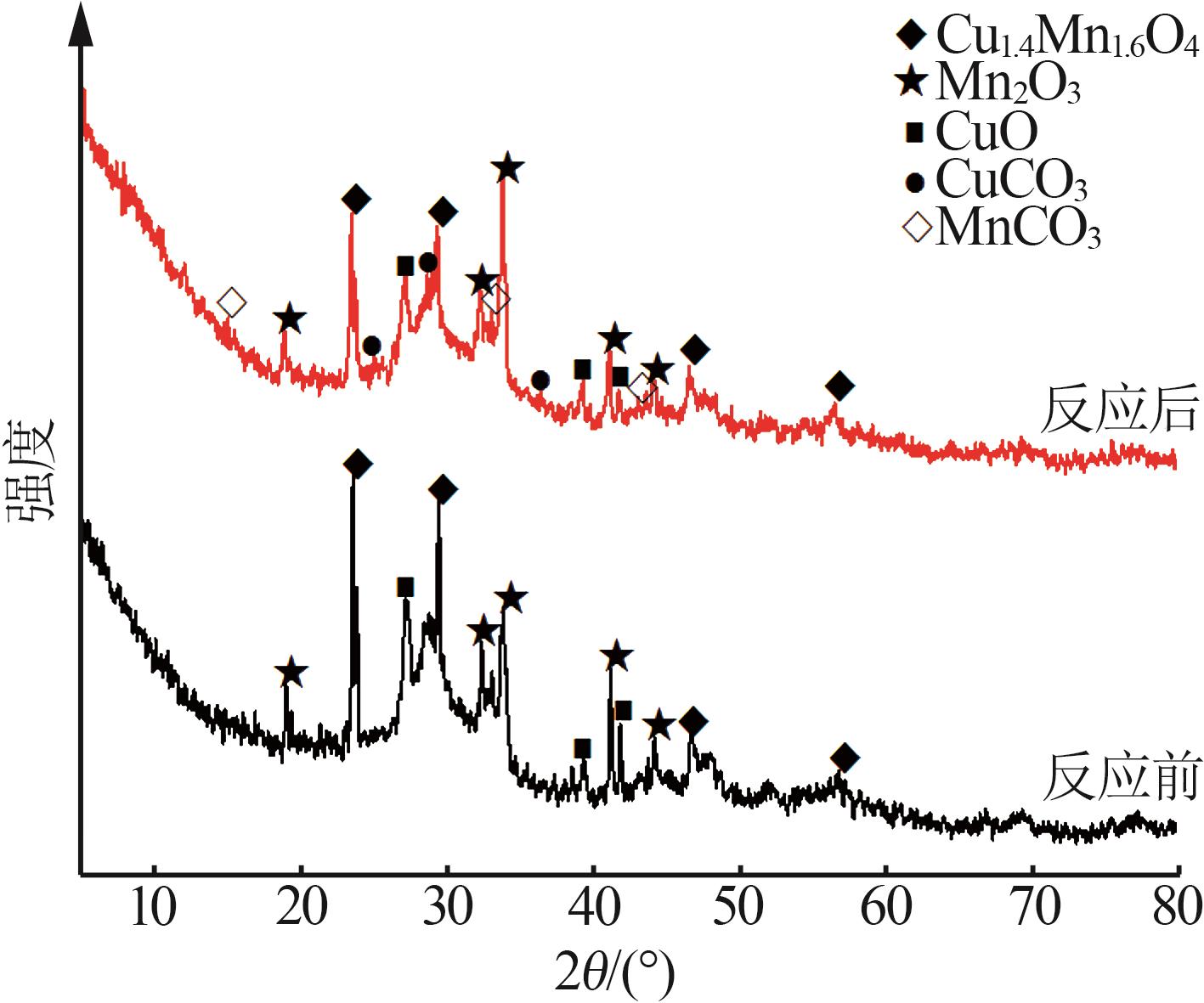
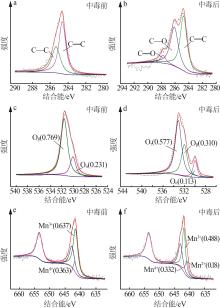
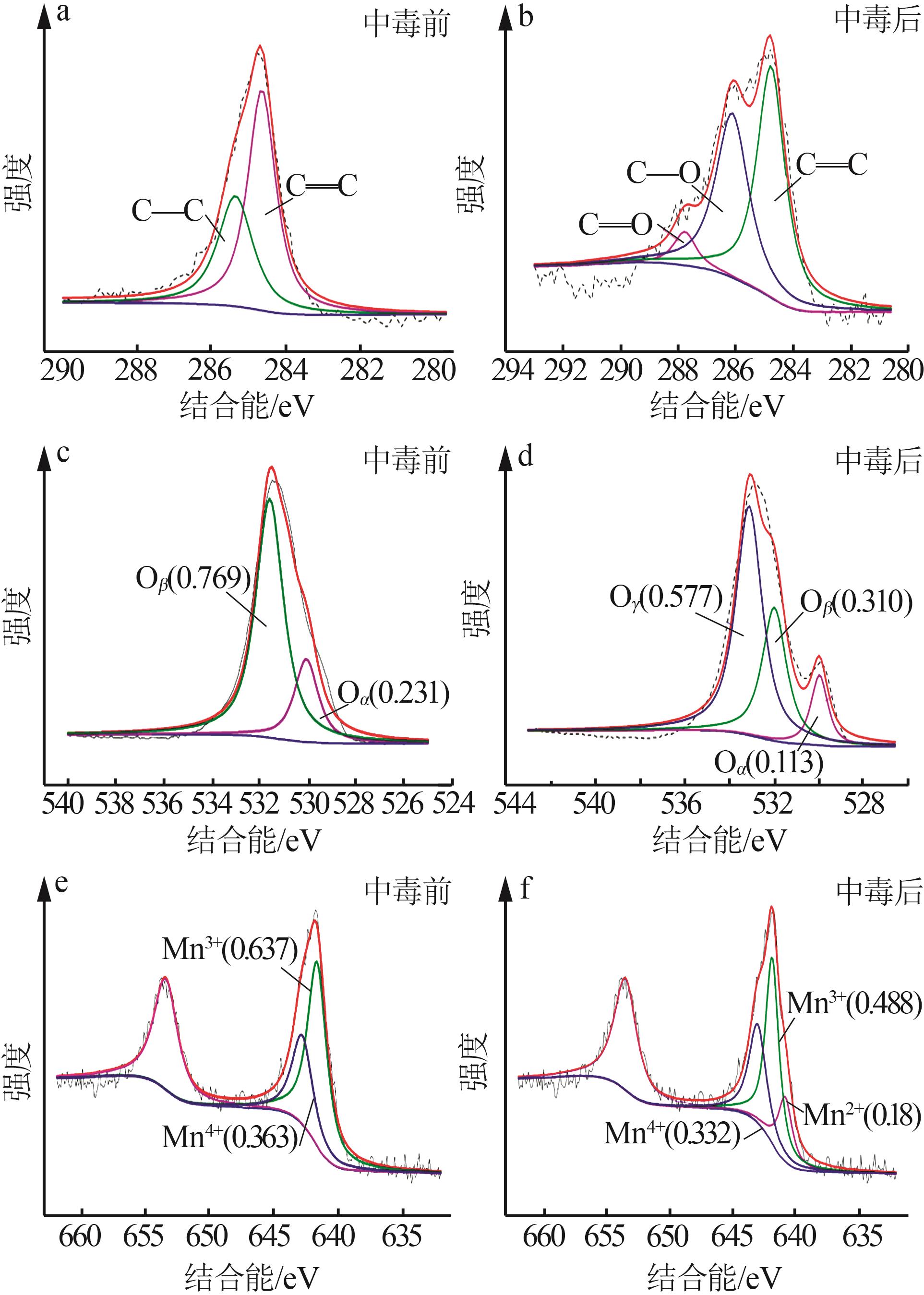
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (6): 142-150.doi: 10.19964/j.issn.1006-4990.2022-0515
• Catalytic Materials • Previous Articles
Study on kinetic model of low concentration formaldehyde catalyzed by copper-manganese oxide
WANG Zhiqiang1,2( ), LIU Xiangcheng1,2, ZHANG Junjie1,2, JIN Wufeng1,2(
), LIU Xiangcheng1,2, ZHANG Junjie1,2, JIN Wufeng1,2( )
)
- 1. Tianjin Key Laboratory of Refrigeration Technology,Tianjin 300134,China
2. School of Mechanical Engineering & Tianjin University of Commerce,Tianjin 300134,China
-
Received:2022-08-29Online:2023-06-10Published:2023-06-14
CLC Number:
Cite this article
WANG Zhiqiang, LIU Xiangcheng, ZHANG Junjie, JIN Wufeng. Study on kinetic model of low concentration formaldehyde catalyzed by copper-manganese oxide[J]. Inorganic Chemicals Industry, 2023, 55(6): 142-150.
share this article
Table 1
Experimental conditions of formaldehyde removal by CuO/MnO2 materials"
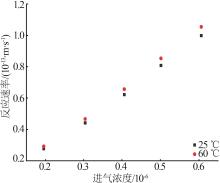
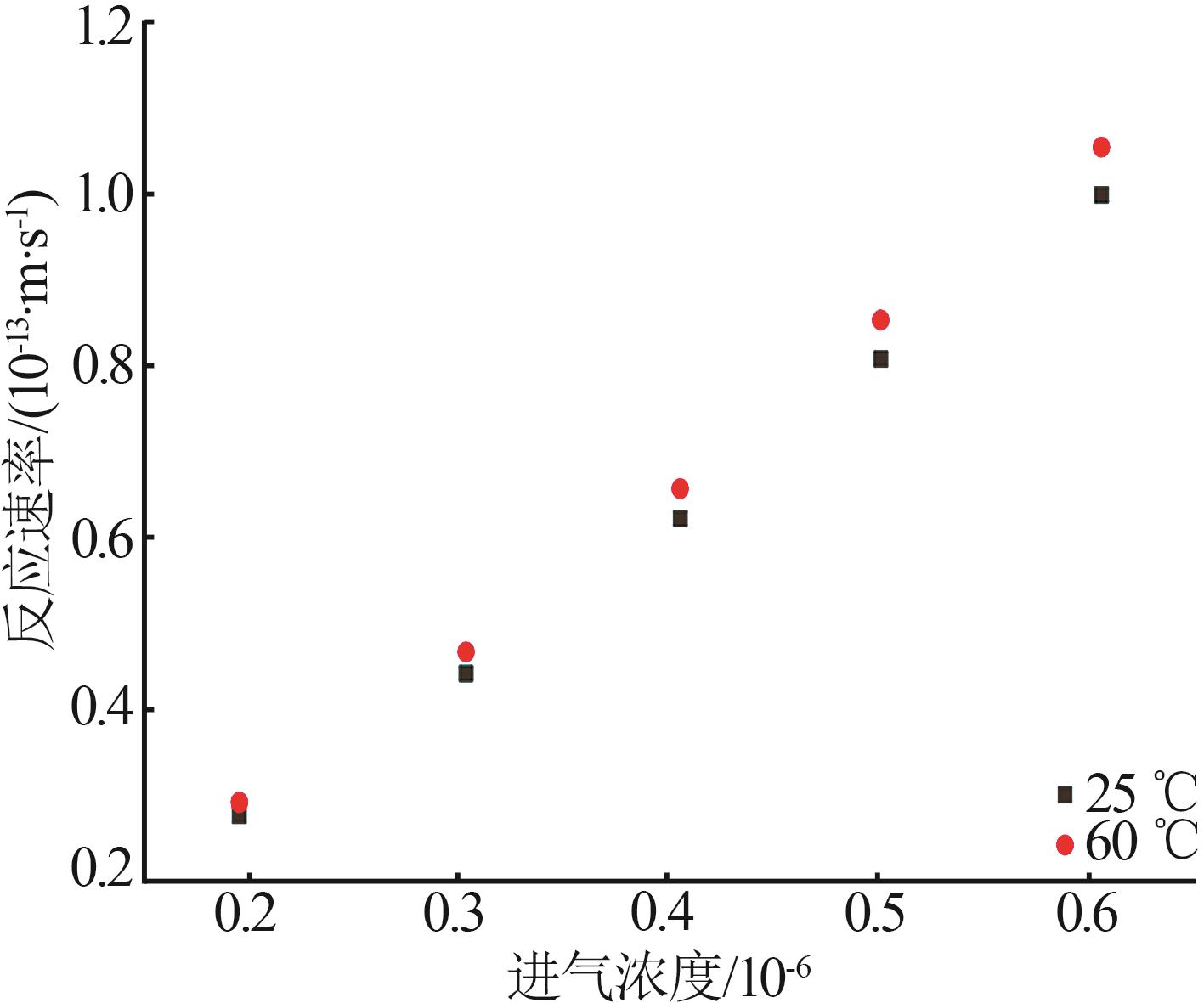
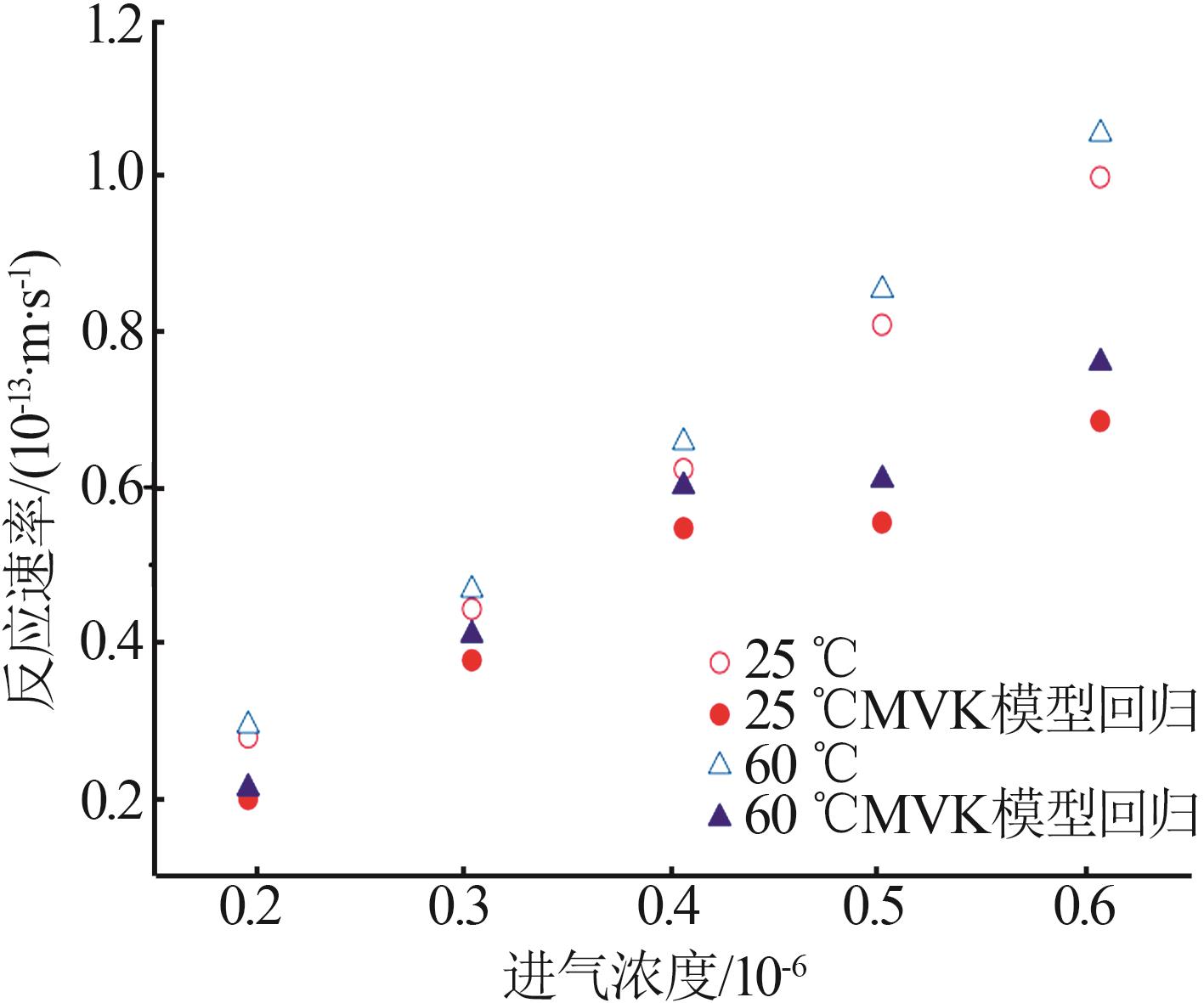
| 催化剂 | 填充厚度/mm | 进气浓度/10-6 | 催化温度/℃ | 初始去除效率/% | 穿透时间/h |
|---|---|---|---|---|---|
| CuO/MnO2-1 | 13 | 0.196 | 25 | 70.7 | 19.9 |
| CuO/MnO2-2 | 13 | 0.304 | 25 | 77.9 | 18.4 |
| CuO/MnO2-3 | 13 | 0.406 | 25 | 83.7 | 19.4 |
| CuO/MnO2-4 | 13 | 0.502 | 25 | 88.5 | 19.4 |
| CuO/MnO2-5 | 13 | 0.304 | 25 | 77.9 | 18.3 |
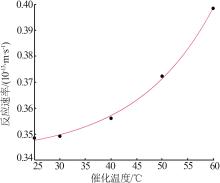
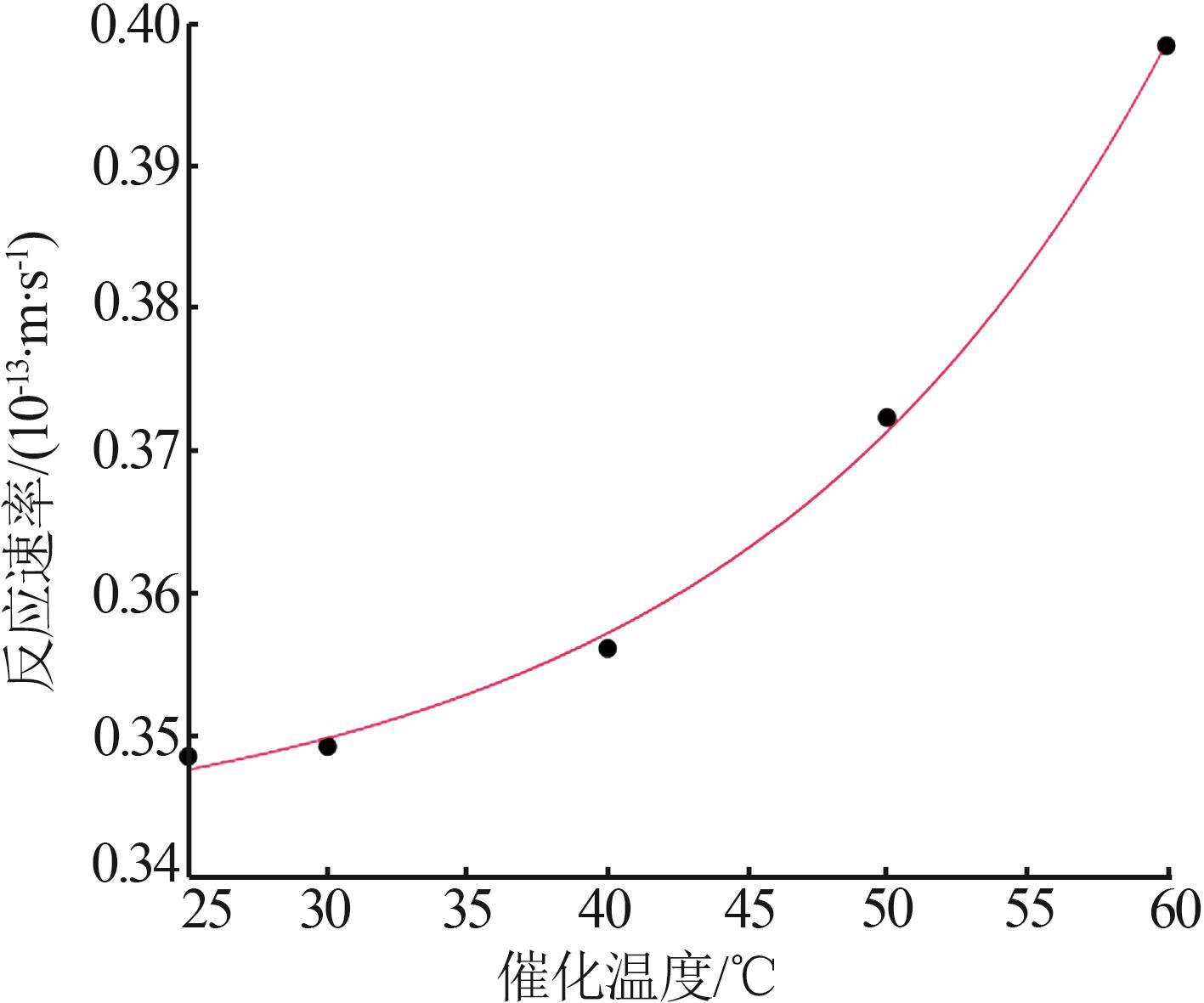
| CuO/MnO2-6 | 13 | 0.304 | 30 | 82.8 | 19.0 |
| CuO/MnO2-7 | 13 | 0.304 | 40 | 84.0 | 18.4 |
| CuO/MnO2-8 | 13 | 0.304 | 50 | 86.8 | 19.4 |
| CuO/MnO2-9 | 13 | 0.304 | 60 | 89.7 | 16.9 |
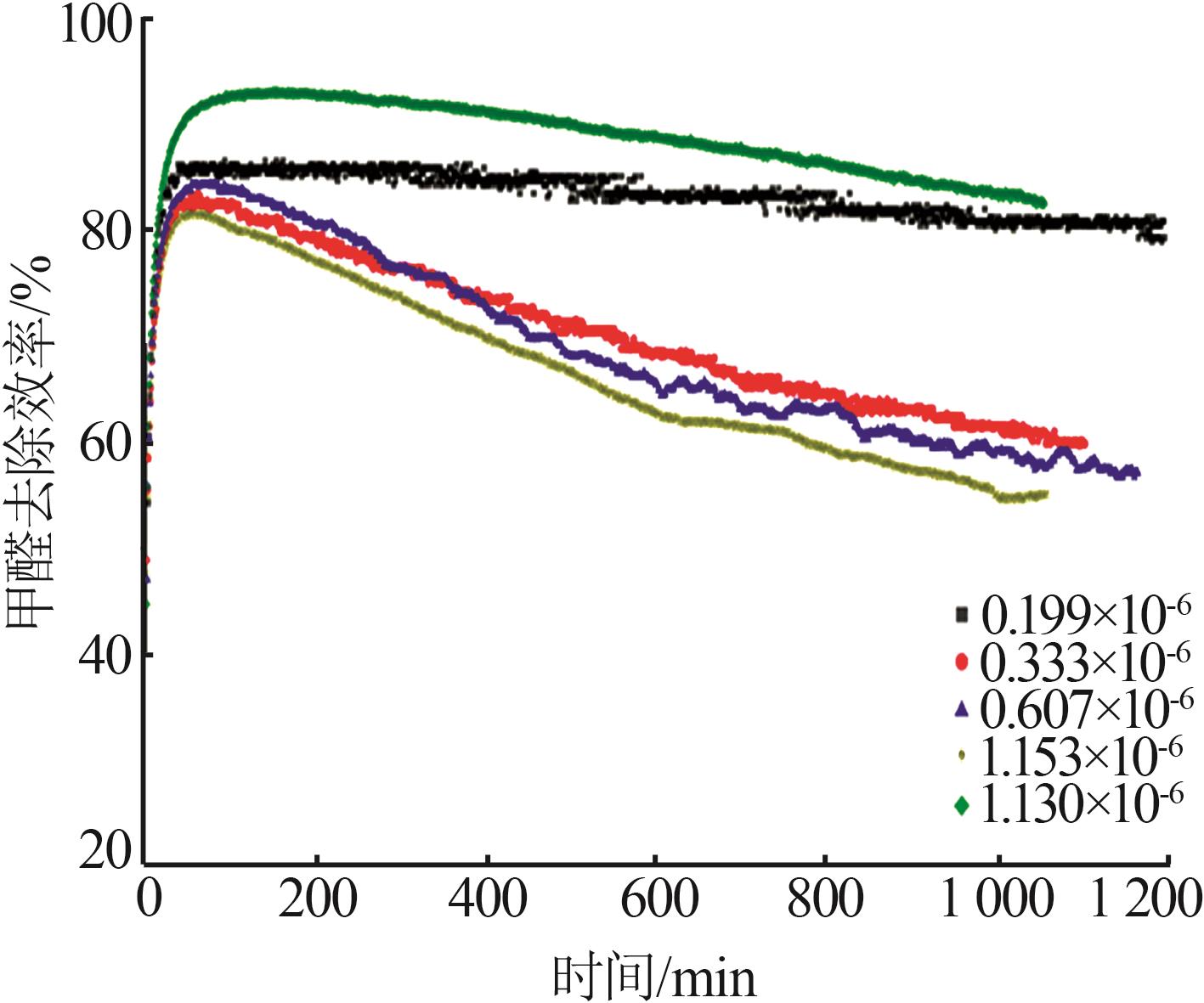
| CuO/MnO2-10 | 3 | 0.199 | 40 | 72.3 | 19.9 |
| CuO/MnO2-11 | 3 | 0.334 | 40 | 72.5 | 18.4 |
| CuO/MnO2-12 | 3 | 0.607 | 40 | 70.9 | 19.4 |
| CuO/MnO2-13 | 3 | 1.130 | 40 | 70.7 | 16.9 |
| CuO/MnO2-14 | 3 | 1.153 | 40 | 71.4 | 17.6 |
| 1 | KAMAL M S, RAZZAK S A, HOSSAIN M M.Catalytic oxidation of volatile organic compounds(VOCs)-A review[J].Atmospheric Environment,2016,140:117-134. |
| 2 | HE Chi, CHENG Jie, ZHANG Xin,et al.Recent advances in the catalytic oxidation of volatile organic compounds:A review based on pollutant sorts and sources[J].Chemical Reviews,2019,119(7):4471-4568. |
| 3 | LIANG Weihui.Long-term indoor formaldehyde variations and health risk assessment in Chinese urban residences following renovation[J].Building and Environment,2021,206:108402. |
| 4 | DAI Xilei, LIU Junjie, YIN Yihui,et al.Modeling and controlling indoor formaldehyde concentrations in apartments:On-site investigation in all climate zones of China[J].Building and Environment,2018,127:98-106. |
| 5 | HUIJBREGTS M A J, ROMBOUTS L J A, RAGAS A M J,et al.Human-toxicological effect and damage factors of carcinogenic and noncarcinogenic chemicals for life cycle impact assessment[J].Integrated Environmental Assessment and Management,2005,1(3):181-244. |
| 6 | World Health Organization/International Agency for Research on Cancer.Formaldehyde,2-butoxyethanol and 1-tert-butoxypropan-2-ol[M]//IARC monographs on the evaluation of carcinogenic risks to humans:Vol 88.Lyon:International Agency for Research on Cancer, 2004. |
| 7 | SALTHAMMER T, MENTESE S, MARUTZKY R.Formaldehyde in the indoor environment[J].Chemical Reviews,2010,110(4):2536-2572. |
| 8 | 中华人民共和国住房和城乡建设部,民用建筑工程室内环境污染控制标准:验收:GB 50325-2020[S].北京:中国计划出版社,2020:17. |
| 9 | TANG Xiaojiang, BAI Yang, DUONG A,et al.Formaldehyde in China:Production,consumption,exposure levels,and health effects[J].Environment International,2009,35(8):1210-1224. |
| 10 | HUANG Kailiang, SUN Wen, FENG Guohui,et al.Indoor air quality analysis of 8 mechanically ventilated residential buildings in northeast China based on long-term monitoring[J].Sustainable Cities and Society,2020,54:101947. |
| 11 | LEE K J, SHIRATORI N, LEE G H,et al.Activated carbon nanofiber produced from electrospun polyacrylonitrile nanofiber as a highly efficient formaldehyde adsorbent[J].Carbon,2010,48(15):4248-4255. |
| 12 | 陈莹,贺军辉,田华,等.铜锰复合氧化物低温催化氧化甲醛的性能研究[J].科技导报,2017,35(23):46-51. |
| CHEN Ying, HE Junhui, TIAN Hua,et al.Copper manganese oxides for formaldehyde catalytic oxidation at low temperature[J].Science & Technology Review,2017,35(23):46-51. | |
| 13 | GUO Jiahong, LIN Chuxia, JIANG Chuanjia,et al.Review on noble metal-based catalysts for formaldehyde oxidation at room temperature[J].Applied Surface Science,2019,475:237- 255. |
| 14 | 左卫元,仝海娟,李健,等.锰氧化物改性离子交换树脂对甲醛的吸附研究[J].无机盐工业,2017,49(1):15-18. |
| ZUO Weiyuan, TONG Haijuan, LI Jian,et al.Adsorption of formaldehyde by manganese oxide modified ion exchange resin[J].Inorganic Chemicals Industry,2017,49(1):15-18. | |
| 15 | MIAO Lei, WANG Jinlong, ZHANG Pengyi.Review on manganese dioxide for catalytic oxidation of airborne formaldehyde[J].Applied Surface Science,2019,466:441-453. |
| 16 | 蔡丽娜.铜锰氧化物制备改性及其CO催化氧化性能研究[D].大连:大连理工大学,2014. |
| CAI Lina. Modification of copper manganese oxide and its catalytic performance of in carbon monoxide oxidation [D].Dalian:Dalian University of Technology,2014. | |
| 17 | 韩旭.热催化氧化法去除室内甲醛的性能及动力学模型研究[D].天津:天津大学,2014. |
| HAN Xu. Performance and kinetic model of thermal catalytic oxidation technology for indoor formaldehyde removal[D].Tianjin:Tianjin University,2014. | |
| 18 | 周微,张耿睿,于海斌,等.MnOx-ZSM-5脱硝催化剂制备的溶剂效应研究[J].无机盐工业,2022,54(8):138-144. |
| ZHOU Wei, ZHANG Gengrui, YU Haibin,et al.Study on solvent effect on preparation of MnOx -ZSM-5 deNOx catalysts[J].Inorganic Chemicals Industry,2022,54(8):138-144. | |
| 19 | HUANG Haibao, XU Ying, FENG Qiuyu,et al.Low temperature catalytic oxidation of volatile organic compounds:A review[J].Catalysis Science & Technology,2015,5(5):2649-2669. |
| 20 | LIOTTA L F.Catalytic oxidation of volatile organic compounds on supported noble metals[J].Applied Catalysis B:Environmental,2010,100(3/4):403-412. |
| 21 | DING Junyan, YANG Yingju, LIU Jing,et al.Catalytic reaction mechanism of formaldehyde oxidation by oxygen species over Pt/TiO2 catalyst[J].Chemosphere,2020,248:125980. |
| 22 | 王志强,张俊杰,刘相成,等.改性活性炭对低浓度二氧化硫吸附动力学模型研究[J].无机盐工业,2022,54(9):69- 76. |
| WANG Zhiqiang, ZHANG Junjie, LIU Xiangcheng,et al.Study on adsorption kinetics model of modified activated carbon on SO2 with low-concentration[J].Inorganic Chemicals Industry,2022,54(9):69-76. | |
| 23 | 刘旺.太阳能热催化降解车内甲醛的影响因素研究[D].天津:天津商业大学,2021. |
| LIU Wang.Research on influencing factors of solar thermal catalytic degradation of formaldehyde in vehicle [D].Tianjin:Tianjin University of Commerce,2021. | |
| 24 | BOYJOO Y, ROCHARD G, GIRAUDON J M,et al.Mesoporous MnO2 hollow spheres for enhanced catalytic oxidation of formaldehyde[J].Sustainable Materials and Technologies,2019,20:e00091. |
| 25 | HAN Zhengyan, WANG Can, ZOU Xuehua,et al.Diatomite-supported birnessite-type MnO2 catalytic oxidation of formaldehyde:Preparation,performance and mechanism[J].Applied Surface Science,2020,502:144201. |
| 26 | LI Jinge, WANG Jinlong, WANG Mingxiao.Birnessite-type manganese oxide on granular activated carbon for formaldehyde removal at room temperature[J].The Journal of Physical Chemistry C,2016,120(42):24121-24129. |
| 27 | LIU Fang, RONG Shaopeng, ZHANG Pengyi,et al.One-step synthesis of nanocarbon-decorated MnO2 with superior activity for indoor formaldehyde removal at room temperature[J].Applied Catalysis B:Environmental,2018,235:158-167. |
| 28 | FANG Ruimei, HUANG Haibao, JI Jian,et al.Efficient MnOx supported on coconut shell activated carbon for catalytic oxidation of indoor formaldehyde at room temperature[J].Chemical Engineering Journal,2018,334:2050-2057. |
| 29 | 张勇,路宁悦,雷涛,等.固相法合成ZIF-8的表征和催化性能研究[J].应用化工,2018,47(5):978-982,987 |
| ZHANG Yong, LU Ningyue, LEI Tao,et al.Characterization and catalytic performances of ZIF-8 synthesized by solid phase synthesis method[J].Applied Chemical Industry,2018,47(5):978-982,987 | |
| 30 | BAI Bingyang, QIAO Qi, LI Junhua,et al.Synthesis of three-dimensional ordered mesoporous MnO2 and its catalytic performance in formaldehyde oxidation[J].Chinese Journal of Catalysis,2016,37(1):27-31. |
| 31 | ZHENG Jiayu, ZHAO Wenkang, WANG Xinxin,et al.Electric-enhanced hydrothermal synthesis of manganese dioxide for the synergistic catalytic of indoor low-concentration formaldehyde at room temperature[J].Chemical Engineering Journal,2020,401:125790. |
| 32 | WANG Jinlong, LI Dandan, LI Peilin,et al.Layered manganese oxides for formaldehyde-oxidation at room temperature:The effect of interlayer cations[J].RSC Advances,2015,5(122):100434-100442. |
| 33 | WANG Zhihui, CHEN Bingbing, CROCKER M,et al.New insights into alkaline metal modified CoMn-oxide catalysts for formaldehyde oxidation at low temperatures[J].Applied Catalysis A:General,2020,596:117512. |
| 34 | 陈玉柱,李济吾,蔡伟建.铜锰镧铈复合催化剂的制备及其降解对二甲苯的机制[J].环境科学学报,2021,41(8):3089- 3099. |
| CHEN Yuzhu, LI Jiwu, CAI Weijian.Preparation of copper-manganese-lanthanum-cerium composite catalyst and its degradation mechanism of p-xylene[J].Acta Scientiae Circumstantiae,2021,41(8):3089-3099. |
| [1] | LIU Zenghui, SONG Bingjie, CHEN He, SUN Peiyong, CHAI Wenzheng, ZHANG Shenghong, FU Songbao, YAO Zhilong. Effect of structure of copper-based catalyst on adsorption and diffusion of mixed solution of unsaturated aldehydes and fatty alcohol [J]. Inorganic Chemicals Industry, 2023, 55(12): 152-158. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||