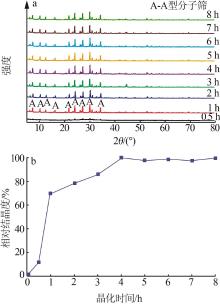
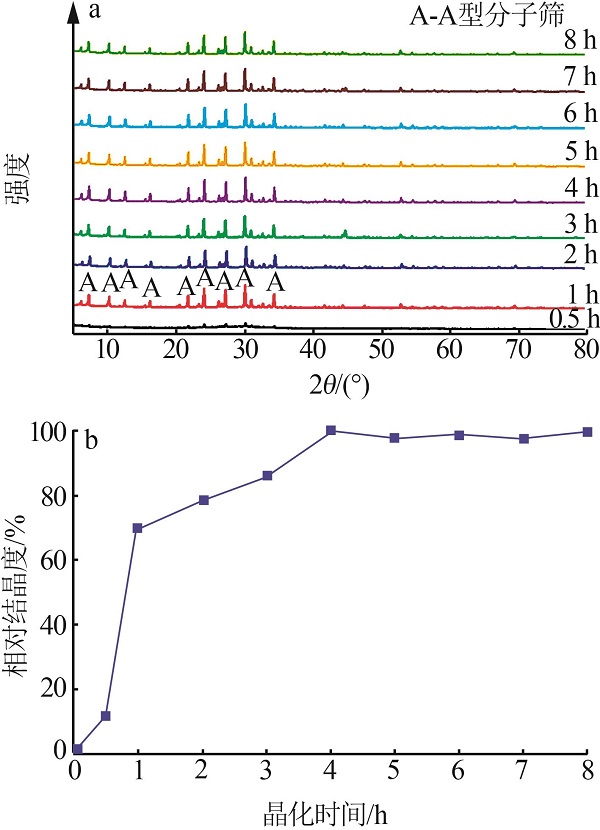
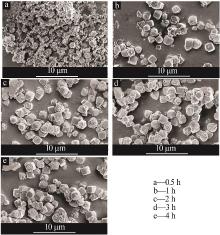
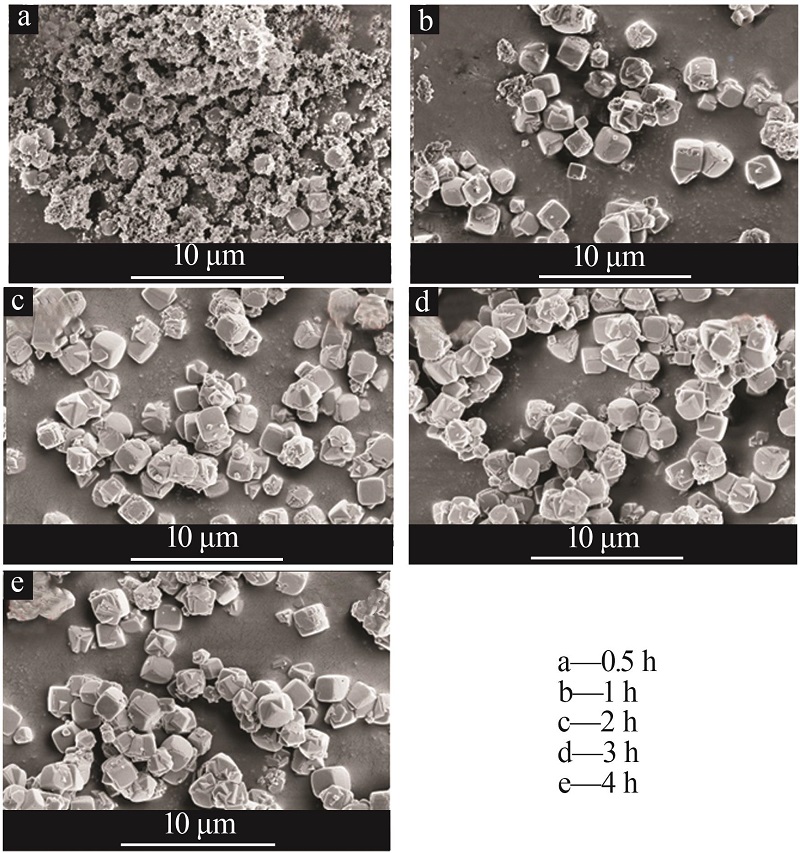
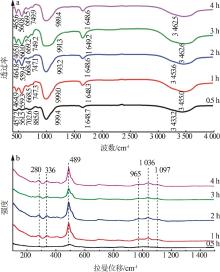
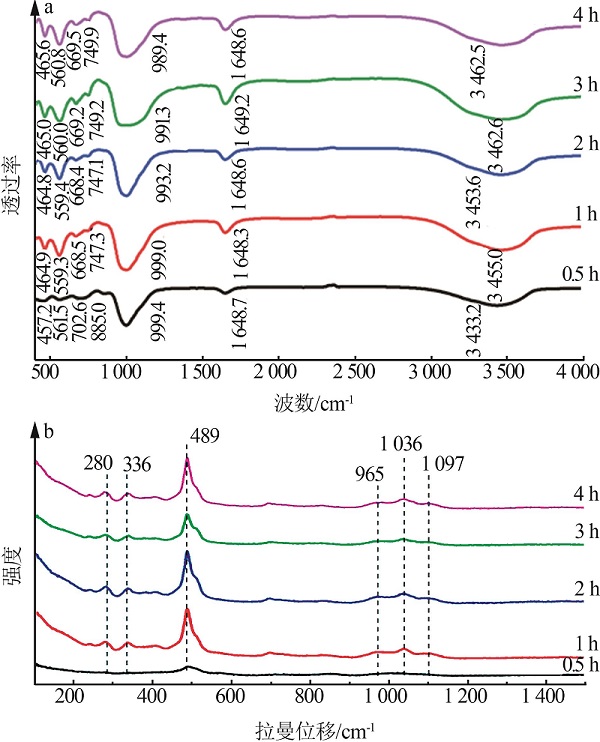
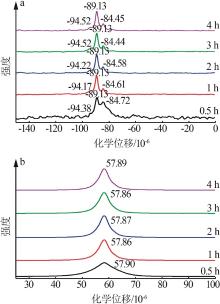
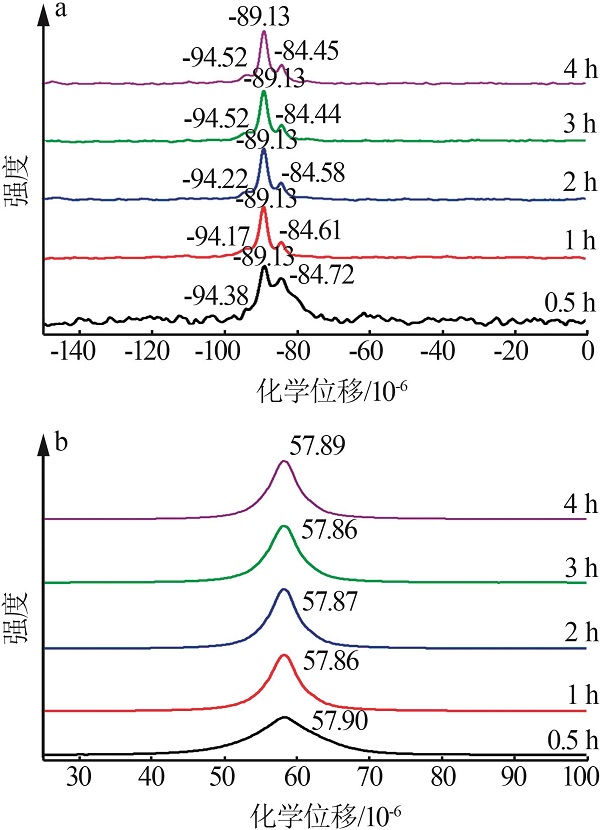
| 1 |
张椿英,陈南春,张小虎,等.A,X,P型分子筛的原位制备与结构控制[J].硅酸盐学报,2014,42(10):1332-1336.
|
|
ZHANG Chunying, CHEN Nanchun, ZHANG Xiaohu, et al.In situ preparation and structure of zeolites A,X and P[J].Journal of the Chinese Ceramic Society,2014,42(10):1332-1336.
|
| 2 |
KUMAR M M, IRSHAD K A, JENA H.Removal of Cs+ and Sr2+ ions from simulated radioactive waste solutions using Zeolite-A synthesized from Kaolin and their structural stability at high pressures[J].Microporous and Mesoporous Materials,2021,312.Doi:10.1016/j.micromeso.2020.110773.
doi: 10.1016/j.micromeso.2020.110773
|
| 3 |
PANGAN N, GALLARDO S, GASPILLO P A, et al.Hydrothermal synthesis and characterization of zeolite A from corn(Zea mays) stover ash[J].Materials(Basel,Switzerland),2021,14(17).Doi:10.3390/ma14174915.
doi: 10.3390/ma14174915
|
| 4 |
SAMANTA N S, BANERJEE S, MONDAL P, et al.Preparation and characterization of zeolite from waste Linz-Donawitz(LD) process slag of steel industry for removal of Fe3+ from drinking water[J].Advanced Powder Technology,2021,32(9):3372-3387.
|
| 5 |
崔家新,王连勇,李尧,等.水淬渣-粉煤灰基4A沸石的制备及性能表征[J].无机盐工业,2022,54(4):135-140.
|
|
CUI Jiaxin, WANG Lianyong, LI Yao, et al.Preparation and properties characterization of water quenching slag-fly ash based 4A zeolite[J].Inorganic Chemicals Industry,2022,54(4):135-140.
|
| 6 |
WANG Binyu, LI Jing, ZHOU Xue, et al.Facile activation of lithium slag for the hydrothermal synthesis of zeolite A with commercial quality and high removal efficiency for the isotope of radioactive 90Sr[J].Inorganic Chemistry Frontiers,2022,9(3):468-477.
|
| 7 |
SU Shuangqing, MA Hongwen, CHUAN Xiuyun.Hydrothermal synthesis of zeolite A from K-feldspar and its crystallization mechanism[J].Advanced Powder Technology,2016,27(1):139-144.
|
| 8 |
KUMAR A, NASKAR M K.Single-step process without organic template for the formation of zeolite A from RHA[J].International Journal of Applied Ceramic Technology,2019,16(4):1525-1532.
|
| 9 |
KUMAR A, NASKAR M K.Custard apple-shaped NaX zeolite with a large surface area derived from rice husk ash by a single-step template-free process[J].Journal of Asian Ceramic Societies,2019,7(3):355-360.
|
| 10 |
REN Limin, LI Caijin, FAN Fengtao, et al.UV-Raman and NMR spectroscopic studies on the crystallization of Zeolite A and a new synthetic route[J].Chemistry-A European Journal,2011,17(22):6162-6169.
|
| 11 |
黄志良,张联盟,刘羽,等.水热法合成4A沸石的相变/纳米聚合生长过程及其机理研究[J].无机材料学报,2005,20(2):401-406.
|
|
HUANG Zhiliang, ZHANG Lianmeng, LIU Yu, et al.Phase transition/nano aggregation growth process of zeolite 4A prepared by hydrothermal synthesis and its mechanism[J].Journal of Inorganic Materials,2005,20(2):401-406.
|
| 12 |
李酽.天然红辉沸石合成A型分子筛(英文)[J].中国民航学院学报,2002(6):30-33.
|
|
LI Yan.Synthesis of zeolite A from natural stellerite[J].Journal of Civil Aviation University of China,2002(6):30-33.
|
| 13 |
申少华,方克明,张术根,等.酸处理红辉沸石-氢氧化钠-铝酸钠-水的水热反应体系A型沸石的形成机理[J].北京科技大学学报,2003,25(6):489-494.
|
|
SHEN Shaohua, FANG Keming, ZHANG Shugen, et al.Formation mechanism of zeolite A in the hydrothermal reaction system of acid-treated stellerite-NaOH-NaAlO2-H2O[J].Journal of University of Science and Technology Beijing,2003,25(6):489-494.
|
| 14 |
高沙沙,陈南春,裴胤昌,等.天然辉沸石为原料制备P型沸石的水热反应条件研究[J].人工晶体学报,2018,47(6):1096-1101.
|
|
GAO Shasha, CHEN Nanchun, PEI Yinchang, et al.Hydrothermal reaction conditions for praparation of zeolite P from natural stilbite as raw material[J].Journal of Synthetic Crystals,2018,47(6):1096-1101.
|
| 15 |
贾敏,杨磊,王永旺.体型化NaA分子筛的制备研究[J].无机盐工业,2021,53(10):98-103.
|
|
JIA Min, YANG Lei, WANG Yongwang.Study on preparation of body-shaped NaA Zeolite[J].Inorganic Chemicals Industry, 2021,53(10):98-103.
|
| 16 |
任英杰,赵永红,张广良.煤矸石两步除铁合成低铁杂质4A沸石[J].无机盐工业,2017,49(1):42-45.
|
|
REN Yingjie, ZHAO Yonghong, ZHANG Guangliang.Synthesis of 4A zeolites with less Fe impurity from gangue after two removaliron stages[J].Inorganic Chemicals Industry,2017,49(1):42-45.
|
| 17 |
PEI Yinchang, ZHONG Yijian, XIE Qinglin, et al.Two-step hydrothermal synthesis and conversion mechanism of zeolite X from stellerite zeolite[J].RSC Advances,2022,12(6):3313-3321.
|
| 18 |
XIAO Yuchen, SHENG Na, CHU Yueying, et al.Mechanism on solvent-free crystallization of NaA zeolite[J].Microporous and Mesoporous Materials,2017,237: 201-209.
|
| 19 |
马淑杰,刘孔凡,崔美珍,等.A型沸石的生成机理[J].高等学校化学学报,1984,5(2):158-162.
|
|
MA Shujie, LIU Kongfan, CUI Meizhen, et al.The machanism of formation of zeolite A[J].Chemical Research in Chinese Universities,1984,5(2):158-162.
|
| 20 |
YANG C S, MORA-FONZ J M, CATLOW C R A.Modeling the nucleation of zeolite A[J].The Journal of Physical Chemistry C,2013,117(47):24796-24803.
|
 ),MO Shengpeng1,XIE Qinglin1,2(
),MO Shengpeng1,XIE Qinglin1,2( ),CHEN Nanchun3,4
),CHEN Nanchun3,4