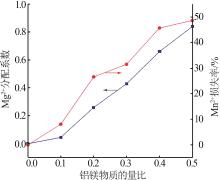
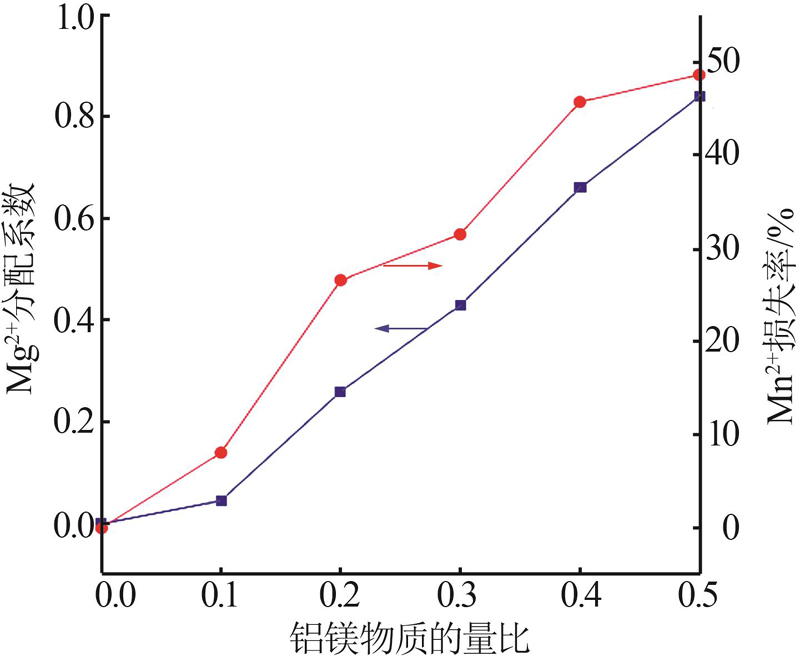
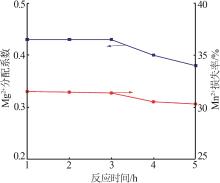
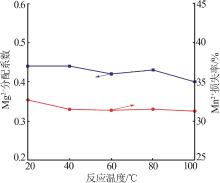
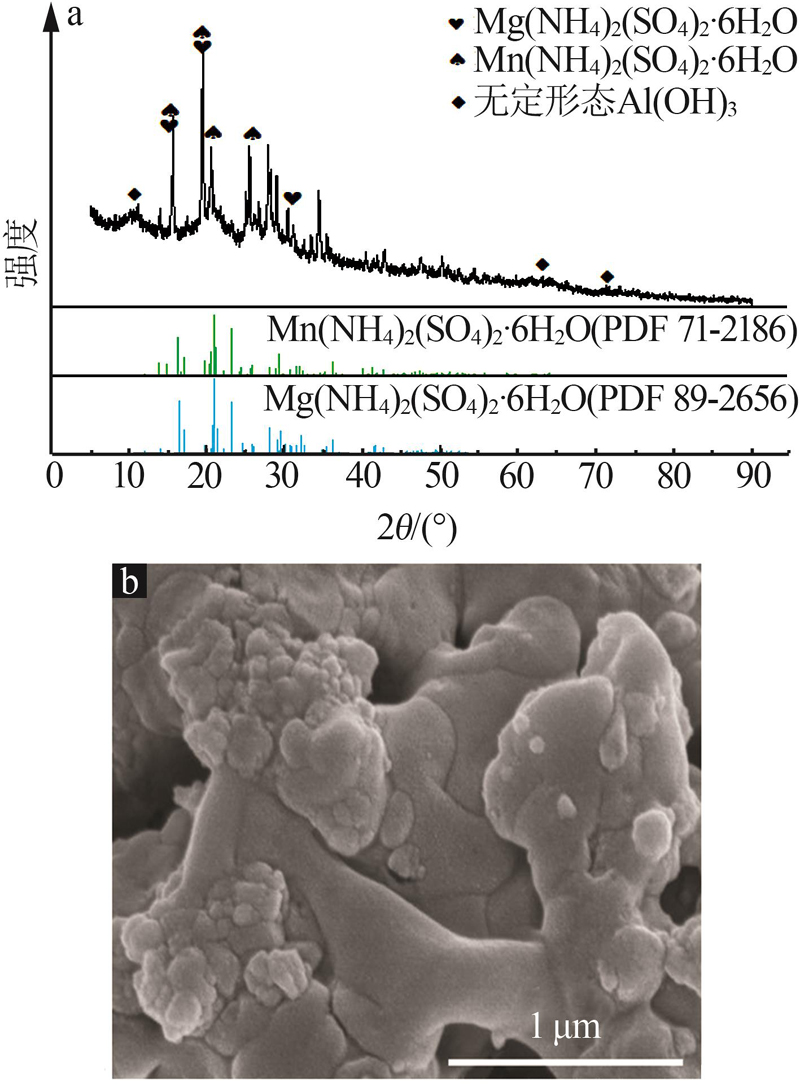
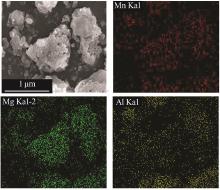
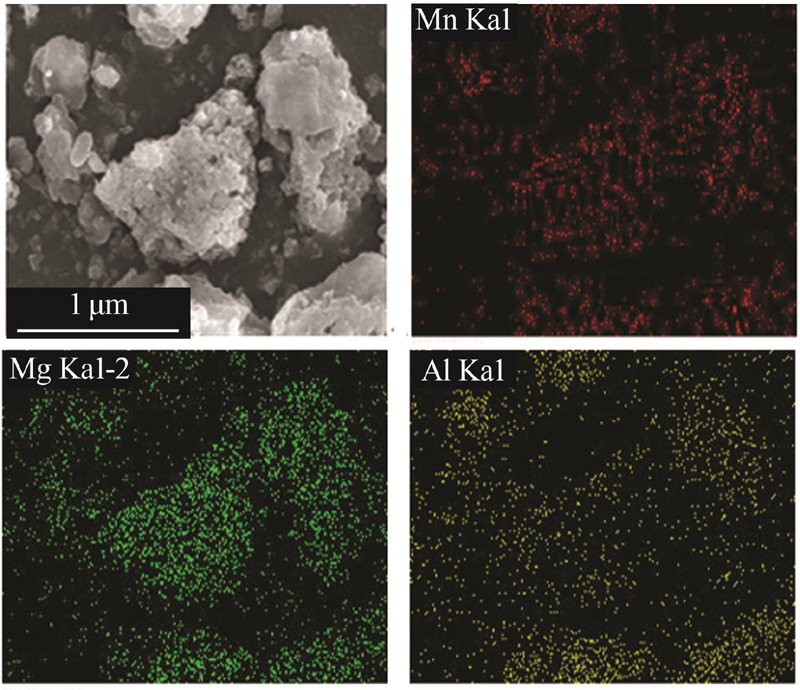
| 1 |
PADHY S K, TRIPATHY B C, ALFANTAZI A.Effect of sodium alkyl sulfates on electrodeposition of manganese metal from sulfate solutions in the presence of sodium metabisulphite[J].Hydrometallurgy,2018,177:227-236.
|
| 2 |
ZHANG Ruirui, MA Xiaotian, SHEN Xiaoxu,et al.Life cycle assessment of electrolytic manganese metal production[J].Journal of Cleaner Production,2020,253.Doi:10.1016/j.jclepro.2019. 119951 .
|
| 3 |
LIN Qingquan, GU Guohua, WANG Hui,et al.Preparation of manganese sulfate from low-grade manganese carbonate ores by sulfuric acid leaching[J].International Journal of Minerals,Metallurgy,and Materials,2016,23(5):491-500.
|
| 4 |
王则奋,黄科林,柳春,等.电解金属锰技术现状及发展趋势[J].大众科技,2019,21(6):26-28.
|
|
WANG Zefen, HUANG Kelin, LIU Chun,et al.The present situation and development tendency of electrolytic manganese[J].Popular Science & Technology,2019,21(6):26-28.
|
| 5 |
LI Changxin, YU Yuan, ZHANG Qingwu,et al.A novel circulation process to effectively produce electrolytic manganese metal(EMM) with low-grade manganese oxide ores and high-sulfur manganese ores[J].Arabian Journal for Science and Engineering,2020,45(9):7561-7572.
|
| 6 |
PENG Xinhong, YU Hongbing, WANG Pan,et al.Production assessment in the electrolytic manganese metal industry in China[J].Metallurgical Research & Technology,2011,108(7/8):437-442.
|
| 7 |
WANG Haifeng, QIN Jitao, TIAN Jiayu,et al.The effects of Mg2+ concentration,(NH4)2SO4 concentration and current density on electrolytic manganese process[J].Materials Research Express,2021,8(2).Doi:10.1088/2053-1591/abe252 .
|
| 8 |
TIAN Jiayu, WANG Haifeng, YOU Xiaoyu,et al.Study on the growth law of manganese in electrolysis process of MnSO4 solution containing magnesium[J].IOP Conference Series:Earth and Environmental Science,2021,668(1).Doi:10.1088/1755-1315/668/1/012085 .
|
| 9 |
蒋文杰,张昭.硫酸锰溶液中镁离子的沉淀行为研究[J].无机盐工业,2014,46(10):34-38.
|
|
JIANG Wenjie, ZHANG Zhao.Precipitation behavior of magnesium ion in manganese sulfate solution[J].Inorganic Chemicals Industry,2014,46(10):34-38.
|
| 10 |
谢子楠,王蛟,沈家国.工业硫酸锰中钙、镁的净化研究[J].无机盐工业,2015,47(5):48-50.
|
|
XIE Zinan, WANG Jiao, SHEN Jiaguo.Research on purification of Ca(Ⅱ) and Mg(Ⅱ) in industrial manganese sulfate[J].Inorganic Chemicals Industry,2015,47(5):48-50.
|
| 11 |
QIN Jitao, WANG Jiawei, WANG Haifeng,et al.Equilibrium distribution of Mg in manganese electrolysis system[J].Materials Research Express,2019,6(9).Doi:10.1088/2053-1591/ab34ac .
|
| 12 |
秦吉涛,王家伟,王海峰,等.Mg2+对硫酸锰电解液理化性质的影响[J].有色金属:冶炼部分,2019(1):12-15,30.
|
|
QIN Jitao, WANG Jiawei, WANG Haifeng,et al.Effect of Mg2+ on physicochemical properties of manganese sulfate electrolyte[J].Nonferrous Metals:Extractive Metallurgy,2019(1):12-15,30.
|
| 13 |
杨洪友.镁、铵在MnSO4溶液中的溶解度及复盐结晶动力学研究[D].贵阳:贵州大学,2020.
|
|
YANG Hongyou.Research on the solubility of magnesium and ammonium in MnSO4 solution and the crystallization kinetics of the double salt[D].Guiyang:Guizhou University,2020.
|
| 14 |
何雨林,李富杰,罗志虹,等.工业硫酸锰高温结晶纯化制备电池级硫酸锰的研究[J].矿冶工程,2019,39(3):85-88.
|
|
HE Yulin, LI Fujie, LUO Zhihong,et al.Preparation of battery-grade manganese sulfate by purification of industrial-grade manganese sulfate with high-temperature crystallization method[J].Mining and Metallurgical Engineering,2019,39(3):85-88.
|
| 15 |
戴冬阳,刘志雄,孙琳,等.萃取法脱除工业级硫酸锰溶液中钙和镁离子[J].吉首大学学报:自然科学版,2016,37(5):55-58,62.
|
|
DAI Dongyang, LIU Zhixiong, SUN Lin,et al.Removal of Ca and Mg ions from industrial manganese sulfate solution by solvent extraction[J].Journal of Jishou University:Natural Sciences Edition,2016,37(5):55-58,62.
|
| 16 |
何婷婷,钱磊,崔静贤,等.氟化法深度脱除工业硫酸锰中钙镁的研究[J].有色金属:冶炼部分,2018(7):1-4.
|
|
HE Tingting, QIAN Lei, CUI Jingxian,et al.Deep removal of Ca and Mg from industrial manganese sulfate with fluorination precipitation[J].Nonferrous Metals:Extractive Metallurgy,2018(7):1-4.
|
| 17 |
陈晓亮,王海峰,王家伟.碳化反沉淀法去除硫酸锰浸出液中钙、镁的研究[J].矿冶工程,2020,40(2):82-85,93.
|
|
CHEN Xiaoliang, WANG Haifeng, WANG Jiawei.Removal of calcium and magnesium from manganese sulfate leaching solution via a reverse precipitation by carbonation[J].Mining and Metallurgical Engineering,2020,40(2):82-85,93.
|
| 18 |
唐境言,石鹏,王宏丹,等.锰电解技术的研究进展综述[J].四川冶金,2020,42(4):9-11.
|
|
TANG Jingyan, SHI Peng, WANG Hongdan,et al.Research progress of manganese electrolysis technology[J].Sichuan Metallurgy,2020,42(4):9-11.
|
| 19 |
田佳瑜.电解条件对阴极锰沉积机制的影响研究[D].贵阳:贵州大学,2021.
|
|
TIAN Jiayu.Study on the effect of electrolysis conditions on cathode manganese deposition mechanism[D].Guiyang:Guizhou University,2021.
|
| 20 |
李昌新,李秋月,喻源,等.以高硫锰矿制备电池用硫酸锰的净化除杂工艺研究[J].无机盐工业,2018,50(7):27-32.
|
|
LI Changxin, LI Qiuyue, YU Yuan,et al.Purification process of electronic grade manganese sulfate prepared by high-sulfur manganese ores[J].Inorganic Chemicals Industry,2018,50(7):27-32.
|
| 21 |
史淼森.五元体系(Li+,Na+,Cs+//Cl-,SO4 2--H2O)及其子体系 298.15 K相平衡研究[D].天津:天津科技大学,2020.
|
|
SHI Miaosen.Phase equilibria of the quinary system and its subsystems containing lithium,sodium,cesium,chloride and sulfate ions at 298.15 K[D].Tianjin:Tianjin University of Science & Technology,2020.
|
| 22 |
刘慧杨,邓志敢,魏昶,等.高温水溶液中铁、锌、镁多元硫酸盐体系的结晶行为[J].中国有色金属学报,2020,30(7):1691-1702.
|
|
LIU Huiyang, DENG Zhigan, WEI Chang,et al.Crystallization behavior of multi-sulfate system of iron,zinc and magnesium in high temperature aqueous solution[J].The Chinese Journal of Nonferrous Metals,2020,30(7):1691-1702.
|
| 23 |
XIE Youhui, LI Qin, ZHAO Xianzhi,et al.Removing and recovering phosphate from poultry wastewater using amorphous cerami-cs[J].Journal of Chemistry,2014.Doi:10.1155/2014/132582 .
|
| 24 |
VARGAS JENTZSCH P, KAMPE B, RÖSCH P,et al.Raman spectroscopic study of crystallization from solutions containing MgSO4 and Na2SO4:Raman spectra of double salts[J].The Journal of Physical Chemistry.A,2011,115(22):5540-5546.
|
| 25 |
GUO Hongfei, CAO Jilin, WANG Jingjie,et al.Phase diagrams of Na2SO4-MgSO4-(NH4)2SO4-H2O system at 25 ℃ and their application[J].Fluid Phase Equilibria,2014,367:79-84.
|
| 26 |
GONG Xuemin, ZHAO Bin, ZHANG Jiayong,et al.Phase diagrams of the Na2SO4-MgSO4-(NH4)2SO4-H2O system at 60 ℃ and their application[J].Journal of Chemical & Engineering Data,2015,60(4):1048-1055.
|
 ), WANG Jiawei1,2(
), WANG Jiawei1,2( ), GOU Bibo1,2, YANG Pan1,2, HE Yue1,2, YANG Chunyuan1,2, WANG Haifeng1,2
), GOU Bibo1,2, YANG Pan1,2, HE Yue1,2, YANG Chunyuan1,2, WANG Haifeng1,2