Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (4): 45-53.doi: 10.19964/j.issn.1006-4990.2022-0358
• Research & Development • Previous Articles Next Articles
Boron species transformation and distribution law of Mg(BO2)2decomposed in LiCl aqueous solution
WEI Fengdan, ZHOU Huan( ), XIA Panping, ZHAO Yun
), XIA Panping, ZHAO Yun
- College of Chemical Engineering and Materials Science,Tianjin University of Science and Technology,Tianjin 300457,China
-
Received:2022-06-13Online:2023-04-10Published:2023-04-13
CLC Number:
Cite this article
WEI Fengdan, ZHOU Huan, XIA Panping, ZHAO Yun. Boron species transformation and distribution law of Mg(BO2)2decomposed in LiCl aqueous solution[J]. Inorganic Chemicals Industry, 2023, 55(4): 45-53.
share this article
Table 1
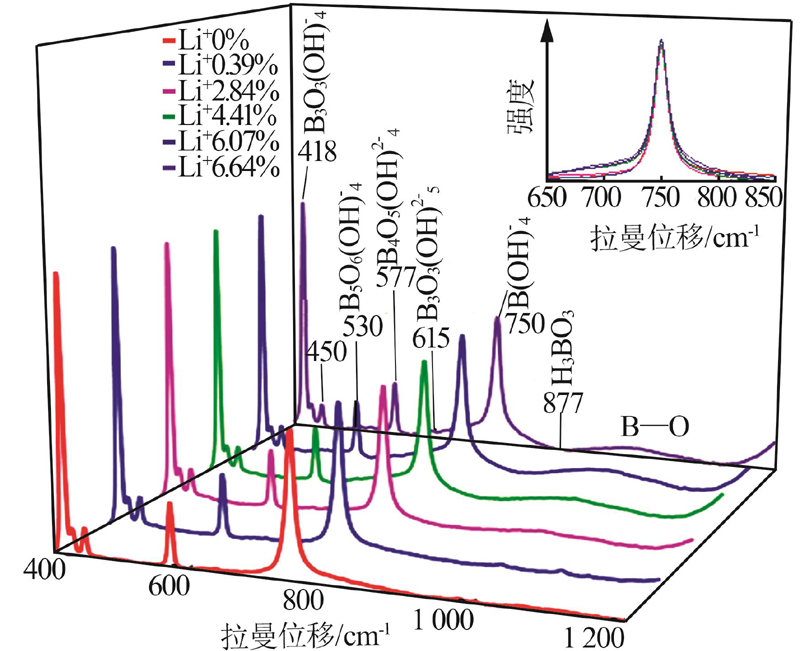
Dominant region and Raman spectra peaks of aqueous boron species"
| 硼物种 | 优势区 pH* | 峰位(本文)/ cm-1 | 峰位(参考文献)/ cm-1 |
|---|---|---|---|
| H3BO3 | <4.0 | 877 | 877[ |
| B(OH)4- | >10 | 750 | 740~755[ |
| B3O3(OH)4- | 7.8 | 418、450 | 440~500[ |
| B3O3(OH)52- | 10.2 | 615 | 610~620[ |
| B4O5(OH)42- | 8.8 | 577 | 577[ |
| B5O6(OH)4- | 6.2 | 530 | 520~530[ |
Table 3
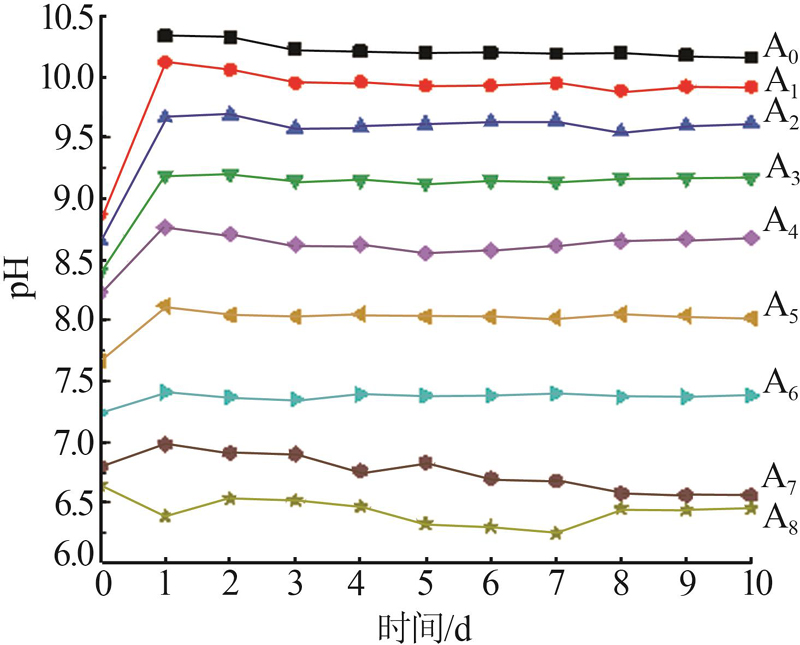
pH change of liquid phase with time for A0~A8 reaction series"
| 组别 | 液相pH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | 2 d | 3 d | 4 d | 5 d | 6 d | 7 d | 8 d | 9 d | 10 d | |
| A0 | 10.34 | 10.33 | 10.23 | 10.21 | 10.20 | 10.20 | 10.19 | 10.20 | 10.18 | 10.16 |
| A1 | 10.12 | 10.06 | 9.95 | 9.96 | 9.93 | 9.93 | 9.95 | 9.89 | 9.92 | 9.95 |
| A2 | 9.66 | 9.69 | 9.58 | 9.59 | 9.61 | 9.63 | 9.64 | 9.55 | 9.60 | 9.62 |
| A3 | 9.19 | 9.20 | 9.15 | 9.15 | 9.13 | 9.15 | 9.14 | 9.17 | 9.17 | 9.07 |
| A4 | 8.76 | 8.71 | 8.62 | 8.62 | 8.55 | 8.57 | 8.61 | 8.65 | 8.67 | 8.68 |
| A5 | 8.12 | 8.04 | 8.03 | 8.05 | 8.03 | 8.03 | 8.02 | 8.05 | 8.04 | 8.02 |
| A6 | 7.41 | 7.37 | 7.35 | 7.39 | 7.38 | 7.38 | 7.40 | 7.37 | 7.37 | 7.38 |
| A7 | 6.98 | 6.91 | 6.90 | 6.76 | 6.83 | 6.70 | 6.68 | 6.58 | 6.56 | 6.64 |
| A8 | 6.39 | 6.53 | 6.52 | 6.47 | 6.33 | 6.30 | 6.25 | 6.45 | 6.44 | 6.45 |
Table 4
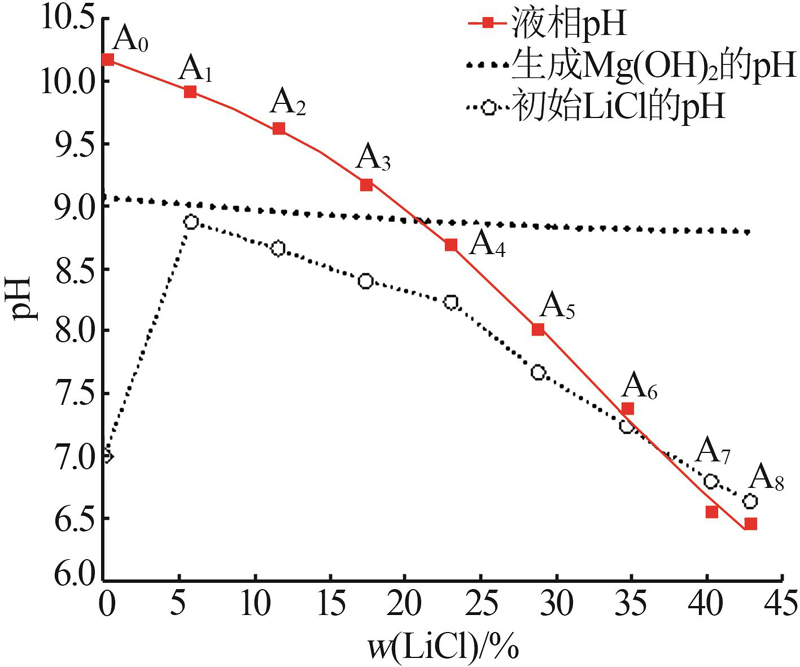
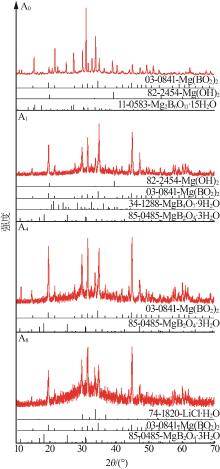
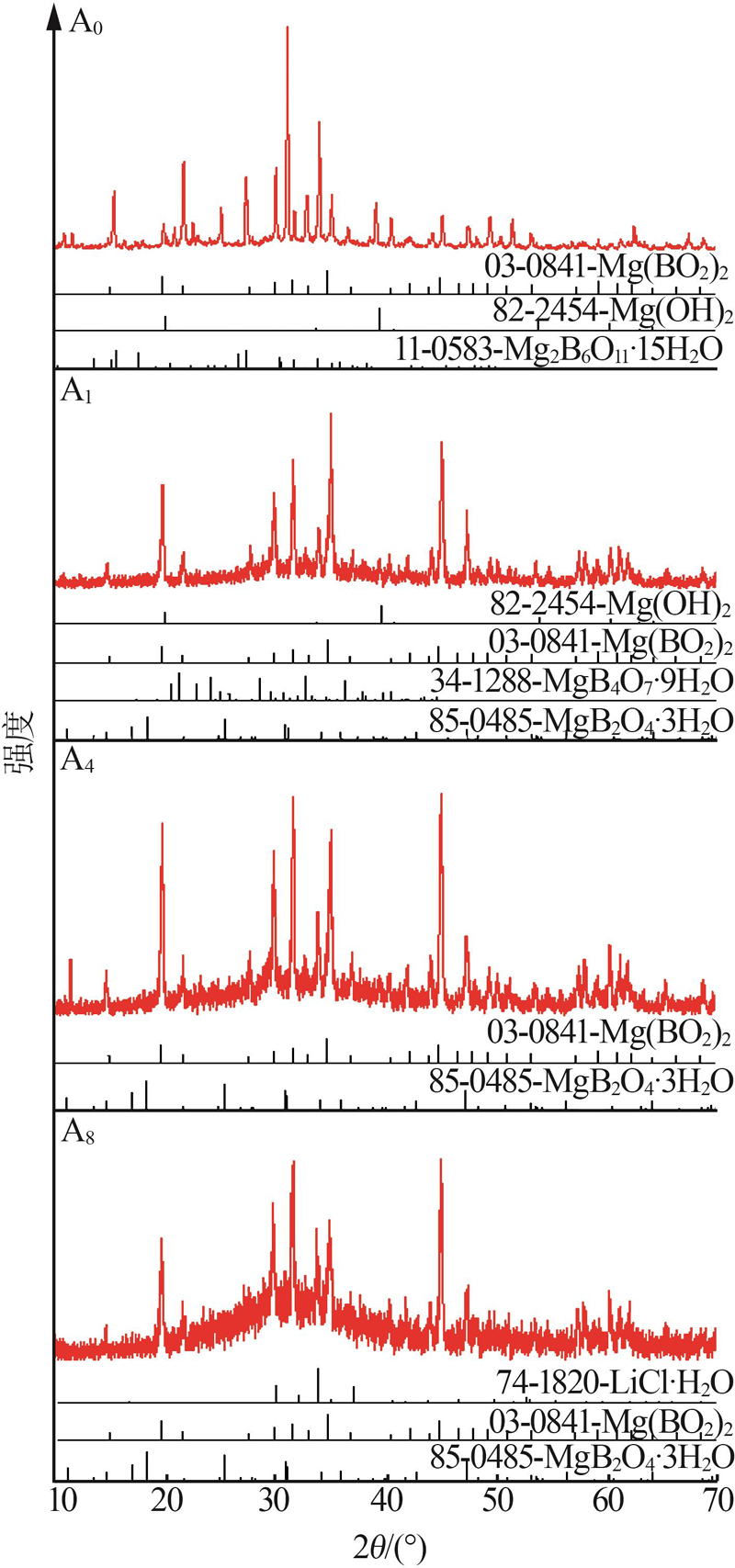
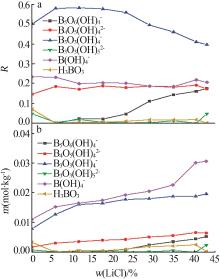
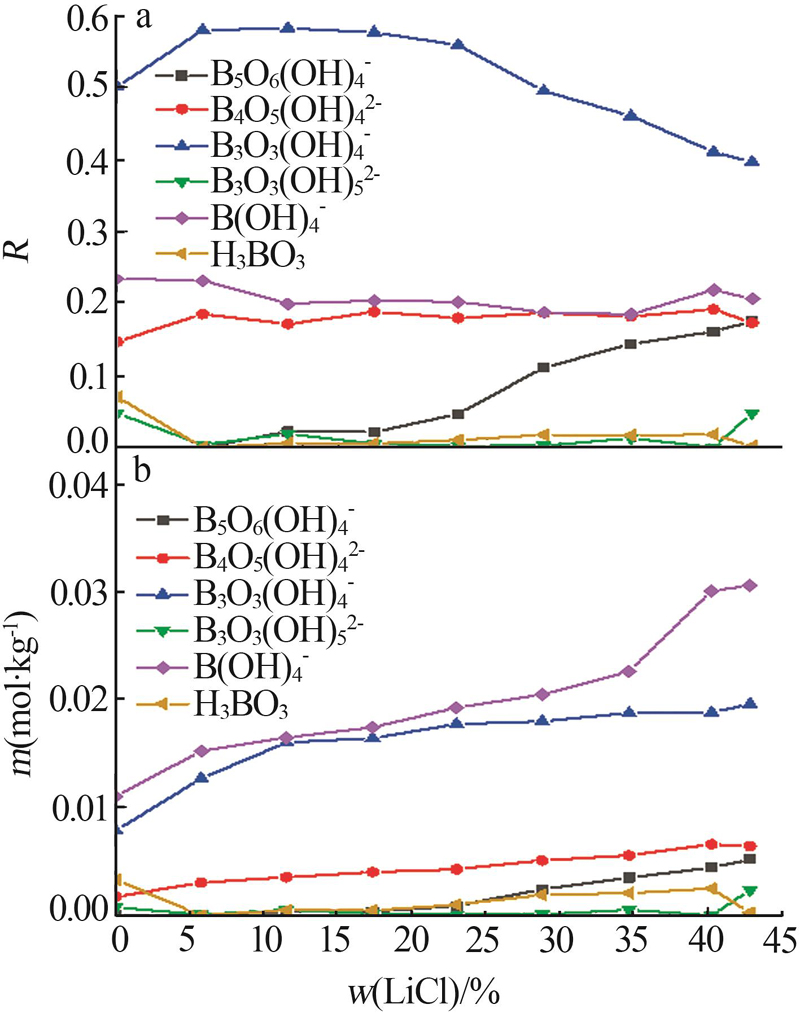
Liquid and solid phase compositions of Mg(BO2)2 decomposed in aqueous LiCl solution"
| 试验编号 | 液相组成质量分数/% | 密度/ (kg·L-1) | pH | 固相组成质量分数/% | 水解固相 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg2+ | Li+ | Cl- | B2O3 | Mg2+ | Li+ | Cl- | B2O3 | ||||||
| 1 d | A0 | 0.01 | 0.00 | 0.00 | 0.18 | 1.001 | 10.34 | 28.05 | 0.00 | 0.00 | 43.12 | — | |
| A1 | 0.01 | 0.10 | 4.75 | 0.24 | 1.035 | 10.12 | 26.18 | 0.45 | 1.24 | 46.86 | — | ||
| A4 | 0.01 | 3.38 | 18.97 | 0.28 | 1.140 | 8.76 | 15.79 | 1.81 | 8.00 | 32.96 | — | ||
| A7 | 0.02 | 5.97 | 33.20 | 0.30 | 1.269 | 6.98 | 16.28 | 2.37 | 8.33 | 32.09 | — | ||
| 2 d | A0 | 0.01 | 0.00 | 0.00 | 0.16 | 1.000 6 | 10.23 | 27.47 | 0.00 | 0.00 | 45.02 | — | |
| A1 | 0.02 | 0.44 | 4.74 | 0.24 | 1.034 4 | 9.95 | 26.25 | 0.34 | 1.58 | 42.24 | — | ||
| A4 | 0.02 | 3.44 | 18.99 | 0.26 | 1.141 9 | 8.62 | 16.57 | 2.33 | 1.67 | 27.73 | — | ||
| A7 | 0.02 | 6.01 | 33.07 | 0.28 | 1.268 8 | 6.90 | 17.26 | 2.58 | 9.99 | 43.38 | — | ||
| 5 d | A0 | 0.02 | 0.00 | 0.00 | 0.17 | 1.000 8 | 10.20 | 35.51 | 0.00 | 0.00 | 55.17 | — | |
| A1 | 0.02 | 0.41 | 4.84 | 0.24 | 1.034 4 | 9.93 | 26.69 | 0.27 | 7.09 | 42.55 | — | ||
| A4 | 0.02 | 3.41 | 19.13 | 0.25 | 1.141 9 | 8.55 | 16.28 | 1.96 | 24.52 | 33.68 | — | ||
| A7 | 0.03 | 6.02 | 33.53 | 0.29 | 1.268 6 | 6.83 | 18.97 | 1.84 | 24.81 | 43.13 | — | ||
| 10 d | A0 | 0.01 | 0.00 | 0.00 | 0.16 | 1.000 2 | 10.16 | 20.06 | 0.00 | 0.00 | 32.25 | S0+S1+S4 | |
| A1 | 0.02 | 0.39 | 4.78 | 0.22 | 1.034 3 | 9.92 | 19.43 | 2.01 | 10.60 | 35.32 | S0+S2+S3+S4 | ||
| A2 | 0.02 | 1.64 | 9.60 | 0.25 | 1.068 3 | 9.62 | 18.86 | 2.00 | 10.84 | 34.67 | S0+S2+S3+S4 | ||
| A3 | 0.02 | 2.84 | 14.28 | 0.25 | 1.104 4 | 9.17 | 19.00 | 2.05 | 10.73 | 33.92 | S0+S2+S3+S4 | ||
| A4 | 0.02 | 3.41 | 18.85 | 0.26 | 1.137 6 | 8.68 | 18.98 | 1.64 | 9.29 | 55.34 | S0+S3 | ||
| A5 | 0.02 | 4.41 | 23.81 | 0.27 | 1.181 5 | 8.02 | 18.29 | 2.23 | 11.16 | 32.48 | S0+S3 | ||
| A6 | 0.02 | 5.10 | 28.53 | 0.28 | 1.223 8 | 7.38 | 18.71 | 2.37 | 11.91 | 29.53 | S0+S3 | ||
| A7 | 0.03 | 6.07 | 32.40 | 0.29 | 1.261 0 | 6.56 | 16.81 | 1.40 | 13.34 | 32.26 | S0+S3 | ||
| A8 | 0.03 | 6.64 | 35.78 | 0.30 | 1.287 0 | 6.45 | 10.64 | 5.35 | 27.88 | 18.31 | S0+S3+S5 | ||
| 1 | 高世扬,宋彭生,夏树屏,等.盐湖化学:新类型硼锂盐湖[M].北京:科学出版社,2007. |
| 2 | 高世扬,符廷进,王建中.盐卤硼酸盐化学:Ⅲ.盐卤在动态蒸发条件下硼酸镁的极限溶解度[J].无机化学学报,1985,1:97-102. |
| GAO Shiyang, FU Tingjin, WANG Jianzhong.Chemistry of borate in salt lake brine—Ⅲ.Maximum solubility of Mg-borate in concentrated salt lake brine[J].Chinese Journal of Inorganic Chemistry,1985,1:97-102. | |
| 3 | 乌志明,崔香梅,郑绵平.高镁含硼盐卤体系反常现象解析[J].无机化学学报,2012,28(1):30-34. |
| WU Zhiming, CUI Xiangmei, ZHENG Mianping.Analysis of abnormal phenomena in high magnesium boron containing salt brine system[J].Chinese Journal of Inorganic Chemistry,2012,28(1):30-34. | |
| 4 | EDWARDS J O.Detection of anionic complexes by pH measurements.I.polymeric borates[J].Journal of the American Chemical Society,1953,75(24):6151-6154. |
| 5 | EVEREST D A, POPIEL W J.Ion-exchange studies of solutions of borates[J].Journal of the Chemical Society(Resumed),1956.Doi:10.1039/JR9560003183 . |
| 6 | INGRI N, ERVASTI A, KROHN C,et al.Equilibrium studies of polyanions.8.On the first equilibrium steps in the hydrolysis of boric acid,a comparison between equilibria in 0.1 M and 3.0 M NaClO4[J].Acta Chemica Scandinavica,1962,16:439-448. |
| 7 | SPESSARD J E.Investigations of borate equilibria in neutral salt solutions[J].Journal of Inorganic and Nuclear Chemistry,1970,32(8):2607-2613. |
| 8 | MESMER R E, BAES C F Jr, SWEETON F H.Acidity measurements at elevated temperatures.Ⅵ.Boric acid equilibriums[J].Inorganic Chemistry,1972,11(3):537-543. |
| 9 | 刘宜娜,杨荣杰,梁嘉香,等.磷核磁共振表征聚磷酸铵聚合度影响因素研究[J].无机盐工业,2018,50(3):66-68. |
| LIU Yina, YANG Rongjie, LIANG Jiaxiang,et al.Characterization of polymerization degree of ammonium polyphosphate by 31P-NMR[J].Inorganic Chemicals Industry,2018,50(3):66-68. | |
| 10 | 王晓燕,张春春.奥美拉唑的核磁共振研究[J].实验科学与技术,2022,20(1):11-15. |
| WANG Xiaoyan, ZHANG Chunchun.The study of nuclear magnetic resonance for omeprazole[J].Experiment Science and Technology,2022,20(1):11-15. | |
| 11 | PENG Jiaoyu, CHEN Jing, DONG Yaping,et al.Investigations on Mg-borate kinetics and mechanisms during evaporation,dilution and crystallization by Raman spectroscopy[J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy,2018,199:367-375. |
| 12 | GE Haiwen, ZHOU Yongquan, LIU Hongyan,et al.Molecular interactions in aqueous solutions of polyborates at different acidity based on the Raman spectroscopy data at 25 ℃[J].Russian Journal of Physical Chemistry A,2017,91(10):1925-1931. |
| 13 | 陈帅,王旭阳,李非,等.强磁场下NH4Cl水溶液结构的拉曼光谱研究[J].光谱学与光谱分析,2021,41(1):116-121. |
| CHEN Shuai, WANG Xuyang, LI Fei,et al.Study of Raman spectroscopy on the structure of NH4Cl aqueous solution under strong magnetic field[J].Spectroscopy and Spectral Analysis,2021, 41(1):116-121. | |
| 14 | 阎波,周桓,李水秀.共聚焦拉曼光谱研究Mg2+和SO4 2-缔合平衡作用[J].过程工程学报,2020,20(9):1063-1073. |
| YAN Bo, ZHOU Huan, LI Shuixiu.Studies on association equilibrium of Mg2+ and SO4 2- by confocal Raman spectroscopy[J].The Chinese Journal of Process Engineering,2020,20(9):1063-1073. | |
| 15 | 毕松岩,仲剑初.四水八硼酸钠的结构及合成机理研究[J].无机盐工业,2019,51(1):43-45,61. |
| BI Songyan, ZHONG Jianchu.Study on synthesis mechanism and structure of disodium octaborate tetrahydrate[J].Inorganic Che-Industry micals,2019,51(1):43-45,61. | |
| 16 | ZHOU Yongquan, FANG Chunhui, FANG Yan,et al.Polyborates in aqueous borate solution:A Raman and DFT theory investigation[J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy,2011,83(1):82-87. |
| 17 | LIU Zhihong, GAO Bo, LI Shuni,et al.Raman spectroscopic analysis of supersaturated aqueous solution of MgO·B2O3-32% MgCl2-H2O during acidification and dilution[J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy,2004,60(13):3125-3128. |
| 18 | 李瑶瑶,周桓,王星帆,等.Mg(BO2)2在MgCl2水溶液中的相平衡与化学平衡[J].无机化学学报,2020,36(8):1421-1429. |
| LI Yaoyao, ZHOU Huan, WANG Xingfan,et al.Phase equilibria and chemical equilibrium of Mg(BO2)2 in MgCl2 aqueous solution[J].Chinese Journal of Inorganic Chemistry,2020,36(8):1421-1429. | |
| 19 | YOSHIDA K, NISHIMOTO S, YAMAGUCHI T.Structural analysis of hydrazinium trifluoroacetate aqueous solution by X-ray diffraction and empirical potential structure refinement modeling in the temperature range of 25 to-125 ℃[J].Journal of Molecular Liquids,2022,353.Doi:10.1016/j.molliq.2022.118802 . |
| 20 | ZHOU Yongquan, YAMAGUCHI T, IKEDA K,et al.Dihydrogen bonds in aqueous NaBD4 solution by neutron and X-ray diffraction[J].The Journal of Physical Chemistry Letters,2020,11(5):1622-1628. |
| 21 | FANG Chunhui, TOSHIO Y, HISANOBU W,et al.X-ray diffraction study on structure of aqueous Mn(NO3)2·6H2O solution[J].Scientific Bulletin,1996,41(16):1353-1358. |
| 22 | PHAM V T, FULTON J L.Contact ion-pair structure in concentrated cesium chloride aqueous solutions:An extended X-ray absorption fine structure study[J].Journal of Electron Spectroscopy and Related Phenomena,2018,229:20-25. |
| 23 | ZHU Fayan, YAMAGUCHI T, YOSHIDA K,et al.Ion hydration and association in aqueous potassium tetrahydroxyborate solutions[J].The Analyst,2020,145(6):2245-2255. |
| 24 | ZHOU Yongquan, HIGA S, FANG Chunhui,et al.B(OH)4 - hydration and association in sodium metaborate solutions by X-ray diffraction and empirical potential structure refinement[J].Physical Chemistry Chemical Physics,2017,19(40):27878-27887. |
| 25 | LI Jun, XIA Shuping, GAO Shiyang.FT-IR and Raman spectroscopic study of hydrated borates[J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy,1995,51(4):519-532. |
| 26 | 王斌,邓小川,史一飞,等.碳酸锂在水和NaCl-KCl溶液体系中溶解度的在线测定[J].无机盐工业,2021,53(7):73-79. |
| WANG Bin, DENG Xiaochuan, SHI Yifei,et al.Online determination of the solubility of lithium carbonate in water and NaCl-KCl solution system[J].Inorganic Chemicals Industry,2021,53(7):73-79. | |
| 27 | 贾永忠.硼酸盐化学—硼酸盐水溶液振动光谱和硼酸盐物理化学[D].兰州:兰州大学,2000. |
| JIA Yongzhong.Borate chemistry-vibration spectrum of borate solution and physical chemistry of borate[D].Lanzhou:Lanzhou University,2000. | |
| 28 | FANG Chunhui, FANG Yan, ZHOU Yongquan,et al.Recent progress on structure of aqueous polyborate solutions[J].Journal of Salt Lake Research,2019,27(2):11-39,2. |
| 29 | ZHANG Tao, LI Dan, MENG Lingzong.Recent progresses on the boron species in aqueous solution:Structure,phase equilibria,metastable zone width(MZW) and thermodynamic model[J].Reviews in Inorganic Chemistry,2021,41(1):49-60. |
| 30 | ZHOU Huan, GU Xiaolong, DAI Yaping,et al.Thermodynamic modeling and phase diagram prediction of salt lake brine systems.I.Aqueous Mg2+-Ca2+-Cl- binary and ternary systems[J].Chinese Journal of Chemical Engineering,2020,28(9):2391-2408. |
| 31 | ZHOU Huan, WU Peng, LI Wenxuan,et al.Thermodynamic modeling and phase diagram prediction of salt lake brine systemsⅡ.Aqueous Li+-Na+-K+-SO4 2- and its subsystems[J].Chinese Journal of Chemical Engineering,2021,34:134-149. |
| 32 | LI Dongdong, ZENG Dewen, YIN Xia,et al.Phase diagrams and thermochemical modeling of salt lake brine systems.Ⅳ.Thermodynamic framework and program implementation for multicomponent systems[J].Calphad,2020,71.Doi:10.1016/j.calphad.2020. 101806 . |
| 33 | CHEN Lele, LI Dan, GUO Yafei,et al.Experimental data and thermodynamic model in the salt-water ternary system(NaBO2+Na2B4O7+H2O) at T=298.15 K and p=0.1 MPa[J].Journal of Chemical & Engineering Data,2019,64(12):5878-5885. |
| 34 | YANG Lan, LI Dan, ZHANG Tao,et al.Thermodynamic phase equilibria in the aqueous ternary system NaCl-NaBO2-H2O:Experimental data and solubility calculation using the Pitzer mo-del[J].The Journal of Chemical Thermodynamics,2020,142.Doi:10.1016/j.jct.2019.106021 . |
| 35 | MAEDA M, HIRAO T, KOTAKA M,et al.Raman spectra of polyborate ions in aqueous solution[J].Journal of Inorganic and Nuclear Chemistry,1979,41(8):1217-1220. |
| 36 | 夏树屏,李军,高世扬.盐卤硼酸盐化学:ⅩⅩⅧ.氯柱硼镁石的激光拉曼光谱[J].无机化学学报,1995,11(2):152-158. |
| XIA Shuping, LI Jun, GAO Shiyang.Laser Raman spectroscopy of chloropinnoite[J].Journal of Inorganic Chemistry,1995,11(2):152-158. | |
| 37 | 张林进,叶旭初.水溶液中硼氧配阴离子的存在形式及影响因素[J].无机盐工业,2008,40(2):4-8. |
| ZHANG Linjin, YE Xuchu.The existing forms and influencing factors of the polyborate anions in aqueous solution[J].Inorganic Chemicals Industry,2008,40(2):4-8. | |
| 38 | 谢先德.硼酸盐晶体结构特征和硼酸盐矿物的结晶化学分类[J].地质论评,1964,10(3):210-223. |
| XIE Xiande.Structural characteristics of borate crystals and crystal chemical classification of borate minerals[J].Geological Review,1964,10(3):210-223. |
| [1] | Xiao Yihan,Cao Jianxin,Liu Fei,Yi Yun. Effect of calcination temperature on physicochemical properties and catalytic performance of MnZnOx [J]. Inorganic Chemicals Industry, 2021, 53(4): 95-100. |
| [2] | YANG Xin-Mei, SUN Ze, HUANG Long, ZHOU Yang, PANG Xu-Yan, SONG Xing-Fu, YU Jian-Guo. Raman spectroscopy of phase change energy storage binary mixed nitrate [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(2): 18-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||