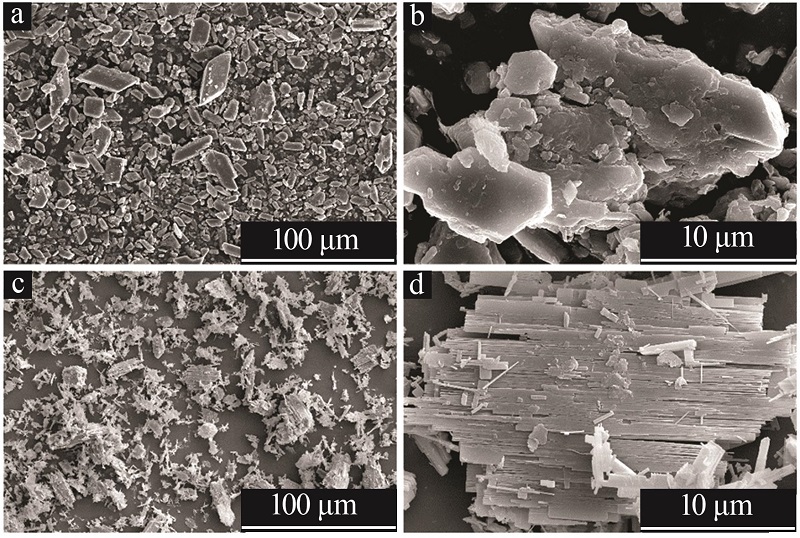
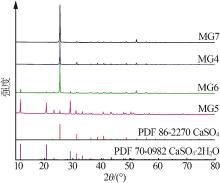
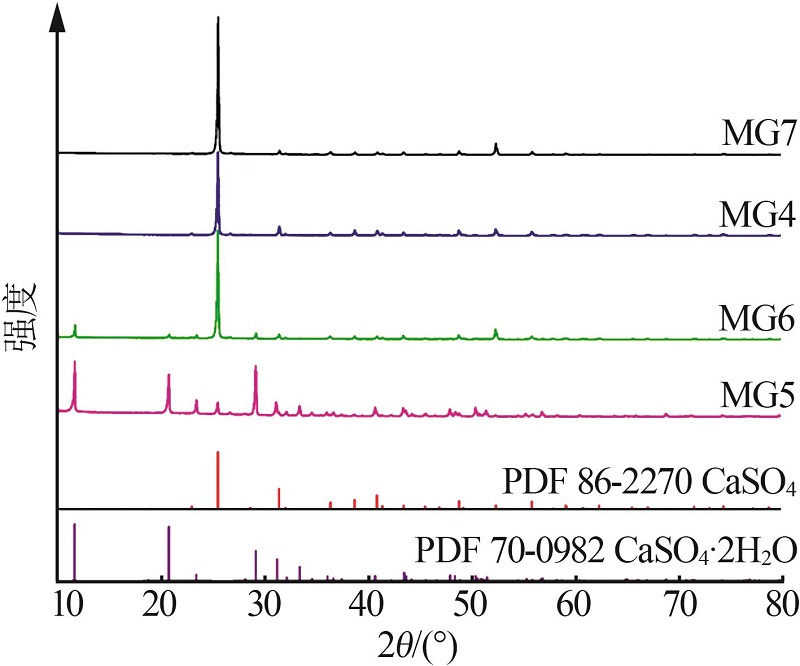
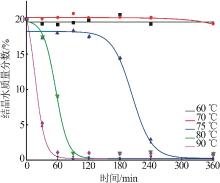
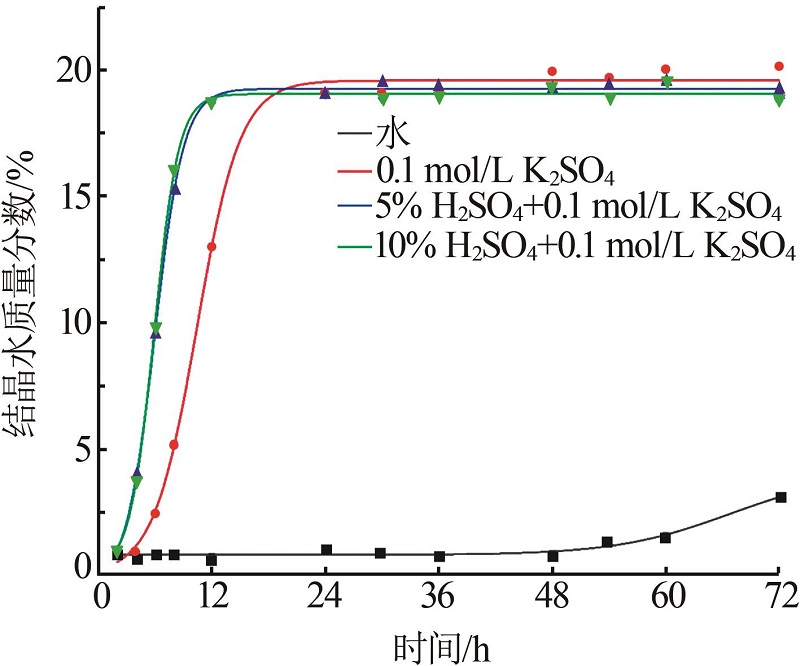
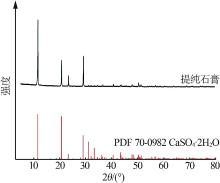
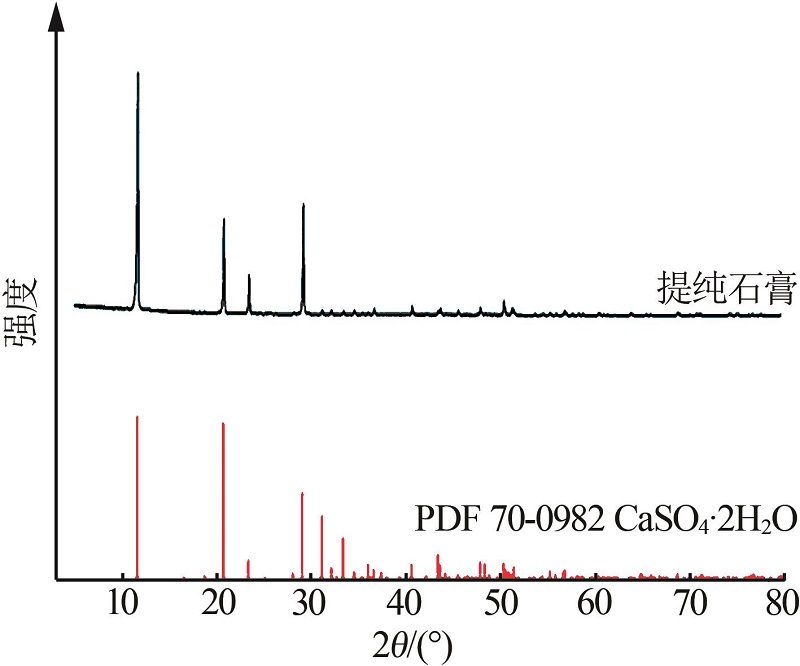
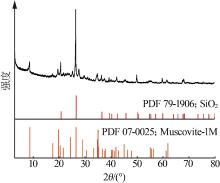
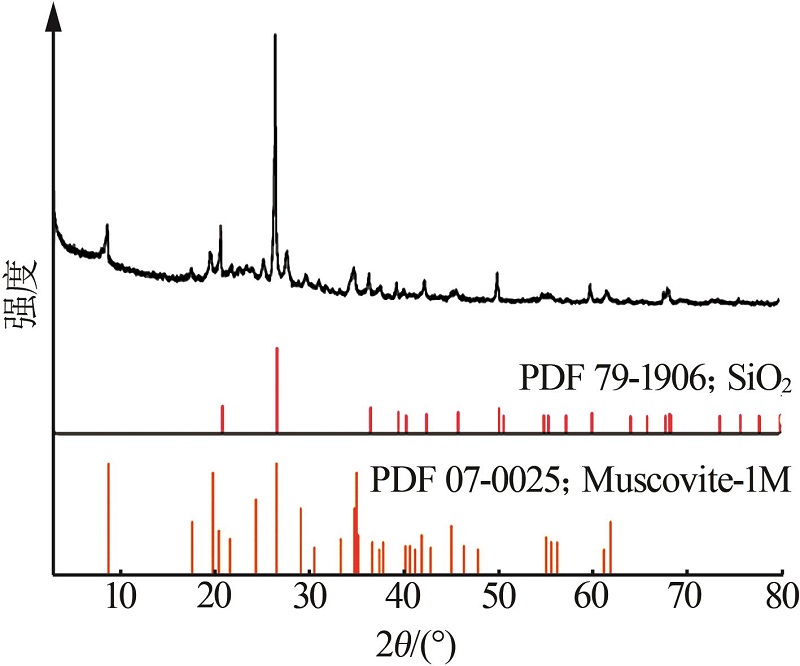
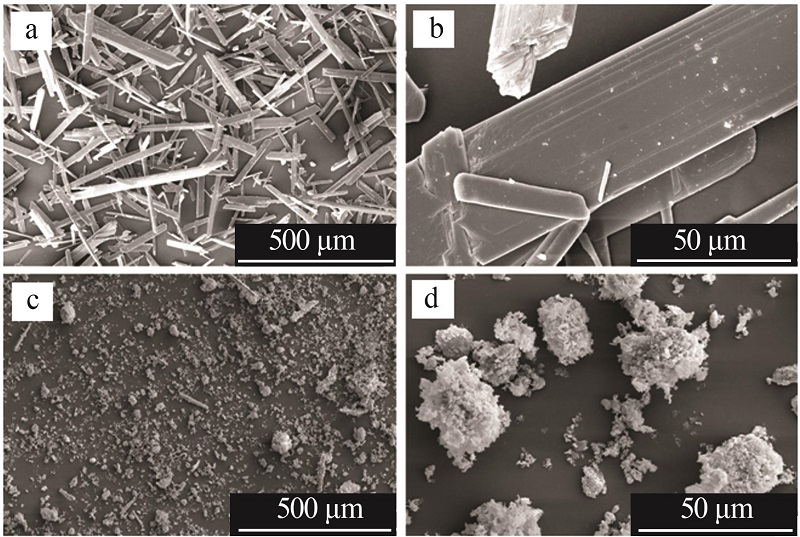
| 1 |
张秀峰,谭秀民,张利珍.钙芒硝矿石的水浸工艺研究[J].矿产保护与利用,2017(4):69-72.
|
|
ZHANG Xiufeng, TAN Xiumin, ZHANG Lizhen.Research on water leaching process of glauberite ore[J].Conservation and Utilization of Mineral Resources,2017(4):69-72.
|
| 2 |
赵宏旭,刘磊.硝盐联产技术在元明粉生产中的应用[J].化工设计通讯,2020,46(9):52-53.
|
|
ZHAO Hongxu, LIU Lei.Application of nitrate combined production technology in the production process of yuanmingfen[J].Che-mical Engineering Design Communications,2020,46(9):52-53.
|
| 3 |
李林,贝鑫.苦卤资源综合利用发展趋势[J].中国盐业,2019(14):48-50.
|
|
LI Lin, BEI Xin.Development trend of comprehensive utilization of bittern resources[J].China Salt Industry,2019(14):48-50.
|
| 4 |
杨大涌,周堃.钙芒硝尾矿的综合利用[J].化工矿物与加工,2020,49(12):53-56.
|
|
YANG Dayong, ZHOU Kun.Comprehensive utilization of glauberite tailings[J].Industrial Minerals & Processing,2020,49(12):53-56.
|
| 5 |
刘林程,左海滨,许志强.工业石膏的资源化利用途径与展望[J].无机盐工业,2021,53(10):1-9.
|
|
LIU Lincheng, ZUO Haibin, XU Zhiqiang.Resource utilization approach of industrial gypsum and its prospect[J].Inorganic Chemicals Industry,2021,53(10):1-9.
|
| 6 |
董益宇.芒硝石膏在水泥生产中的应用[J].四川建材,2017,43(10):24-26.
|
|
DONG Yiyu.Application of glauber's salt gypsum in cement production[J].Sichuan Building Materials,2017,43(10):24-26.
|
| 7 |
张利珍,吕子虎,张永兴,等.磷石膏提质降杂实验研究[J].无机盐工业,2021,53(6):171-173,184.
|
|
ZHANG Lizhen, Zihu LÜ, ZHANG Yongxing, et al.Experimental study on improving quality and reducing impurity of phosphogypsum[J].Inorganic Chemicals Industry,2021,53(6):171-173,184.
|
| 8 |
张巨松,高飞,安会勇,等.低品位石膏提纯与增白实验研究[J].非金属矿,2004,27(6):44-46.
|
|
ZHANG Jusong, GAO Fei, AN Huiyong, et al.Experimental research on purification and whitening of low-grade gypsum[J].Non-Metallic Mines,2004,27(6):44-46.
|
| 9 |
王进明,董发勤,王肇嘉,等.磷石膏浮选增白净化新工艺研究[J].非金属矿,2019,42(5):1-5.
|
|
WANG Jinming, DONG Faqin, WANG Zhaojia, et al.Study on new technology of phosphogypsum whitening and purification by flotation[J].Non-Metallic Mines,2019,42(5):1-5.
|
| 10 |
ROGERS K J, OWENS F C.Purification of FGD gypsum product:US,5215672[P].1993-06-01.
|
| 11 |
GRONE D.Process of purifying gypsum:US,5500197[P].1996-03-19.
|
| 12 |
赵志曼,刘子瑜,李帅,等.磷石膏炒制-酸浸法脱色增白研究[J].低温建筑技术,2016,38(5):9-11.
|
|
ZHAO Zhiman, LIU Ziyu, LI Shuai, et al.Research on decolorization and whitening of phosphogypsum frying-acid leaching me-thod[J].Low Temperature Architecture Technology,2016,38(5):9-11.
|
| 13 |
张伟卓,赵斌,陈学青,等.脱硫石膏晶体提纯脱色的研究[J].人工晶体学报,2015,44(4):1069-1076,1083.
|
|
ZHANG Weizhuo, ZHAO Bin, CHEN Xueqing, et al.Decoloration and purification of FGD gypsum crystal[J].Journal of Synthetic Crystals,2015,44(4):1069-1076,1083.
|
| 14 |
卢静昭,赵斌,陈学青,等.利用脱硫石膏制备高品质二水石膏[J].人工晶体学报,2016,45(1):97-103.
|
|
LU Jingzhao, ZHAO Bin, CHEN Xueqing, et al.Preparation of the high-quality gypsum by dihydrate FGD[J].Journal of Synthetic Crystals,2016,45(1):97-103.
|
| 15 |
杨新亚,喻德高,王锦华.硫酸钾对无水硫酸钙水化激发机理研究[J].沈阳建筑大学学报:自然科学版,2008,24(1):104-107.
|
|
YANG,Xinya, YU Degao, WANG Jinhua.Research on hydration mechanism of calcium sulfate anhydrite activated by potassium sulfate[J].Journal of Shenyang Jianzhu University:Natural Science,2008,24(1):104-107.
|
| 16 |
喻德高.无水硫酸钙水化机理及应用研究[D].武汉:武汉理工大学,2007.
|
|
YU Degao.Research on the hydration machanism and application of anhydrous calcium sulphate[D].Wuhan:Wuhan University of Technology,2007.
|
 ),LI Jun(
),LI Jun( ),ZHENG Zhuochao
),ZHENG Zhuochao