Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (9): 150-157.doi: 10.19964/j.issn.1006-4990.2022-0009
• Catalytic Materials • Previous Articles Next Articles
Effect of sulfate on photocatalytic activity of polymerized carbon nitride
MA Bingxiang1( ),SHEN Yunxia2,LI Na1,LI Min1,WEI Yaoyi1,ZHAO Yu1(
),SHEN Yunxia2,LI Na1,LI Min1,WEI Yaoyi1,ZHAO Yu1( )
)
- 1. College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, China
2. Yantai High?tech Industrial Development Zone Environmental Law Enforcement Brigade
-
Received:2022-01-06Online:2022-09-10Published:2022-09-22 -
Contact:ZHAO Yu E-mail:m1078077097@163.com;zhaoyusky781215@163.com
CLC Number:
Cite this article
MA Bingxiang,SHEN Yunxia,LI Na,LI Min,WEI Yaoyi,ZHAO Yu. Effect of sulfate on photocatalytic activity of polymerized carbon nitride[J]. Inorganic Chemicals Industry, 2022, 54(9): 150-157.
share this article
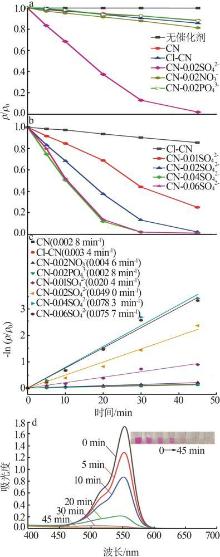
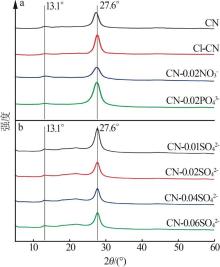
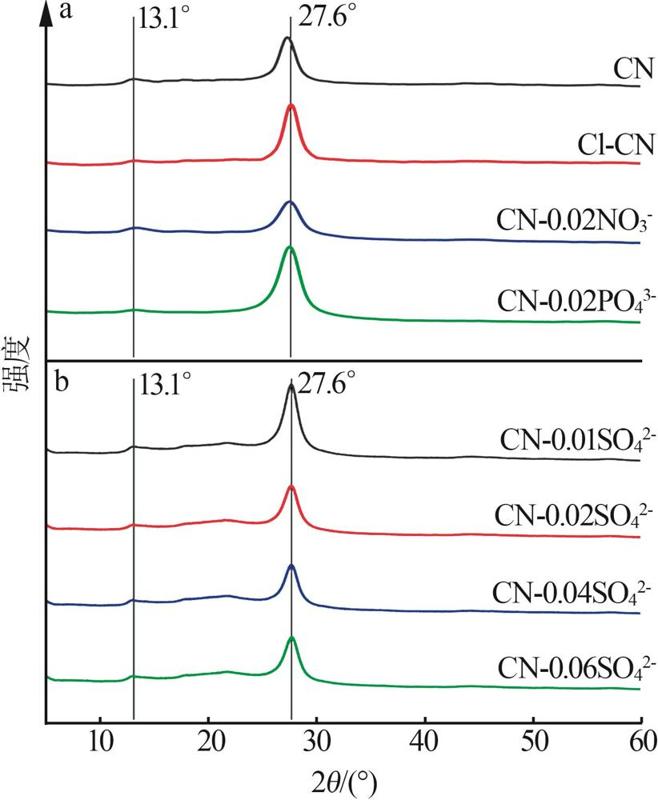
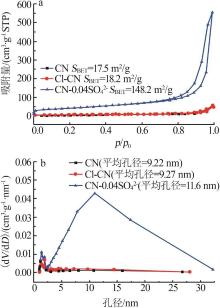
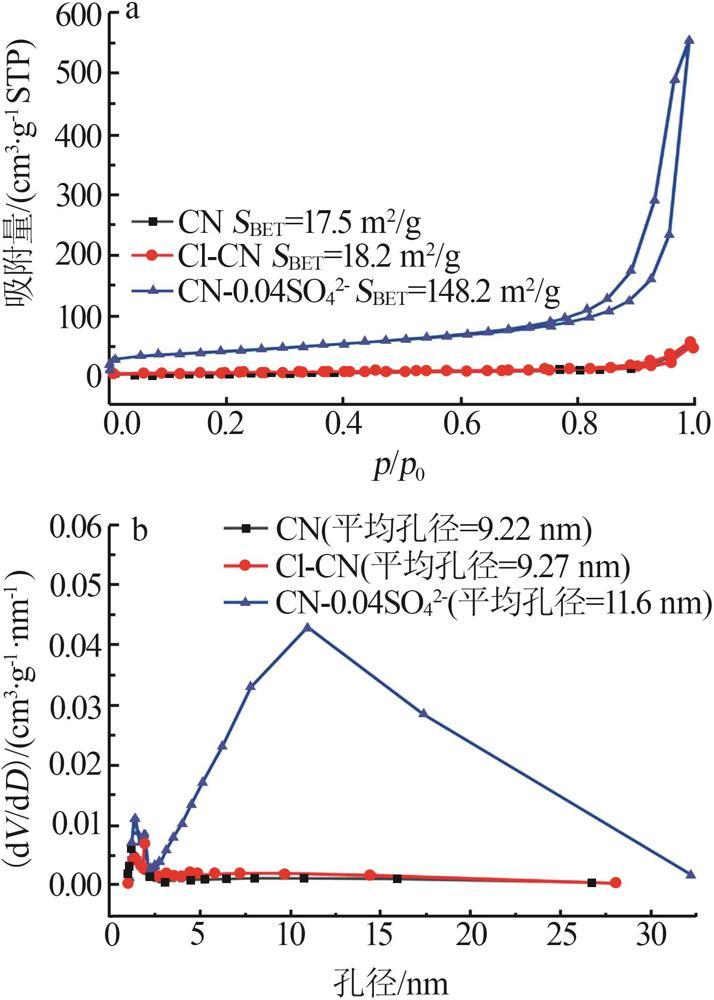
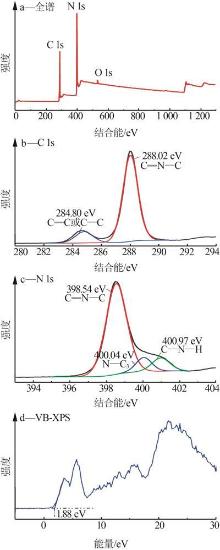
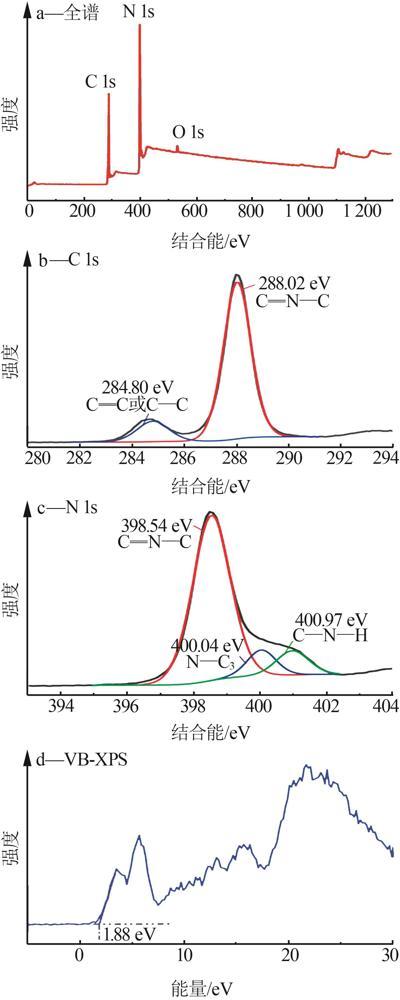
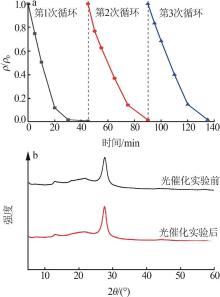
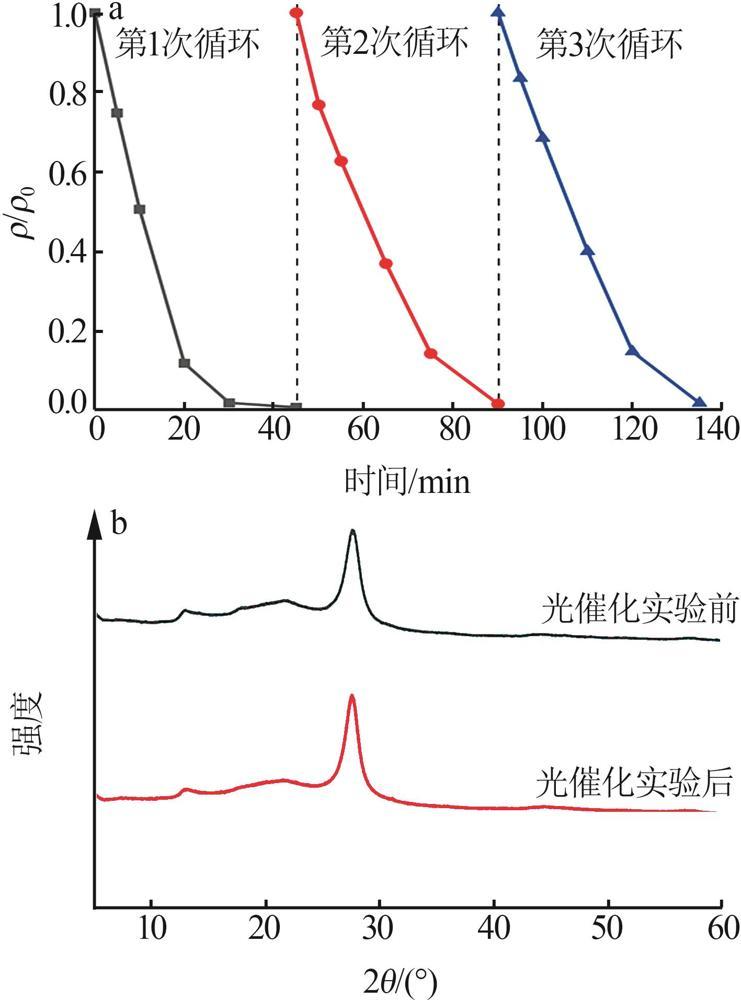
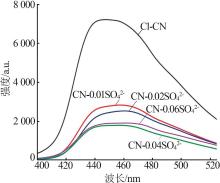
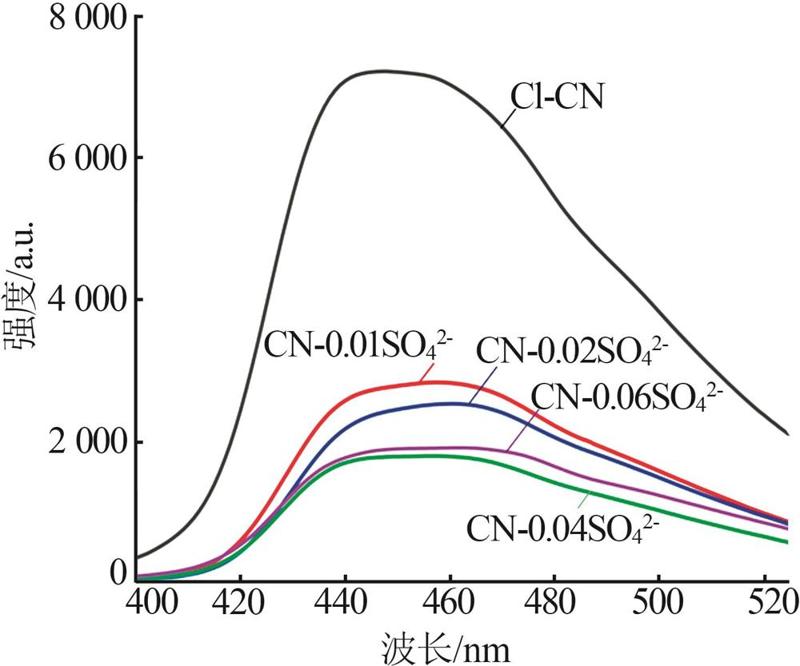
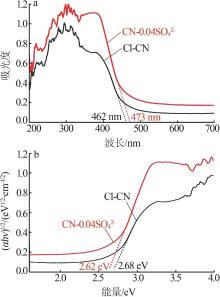
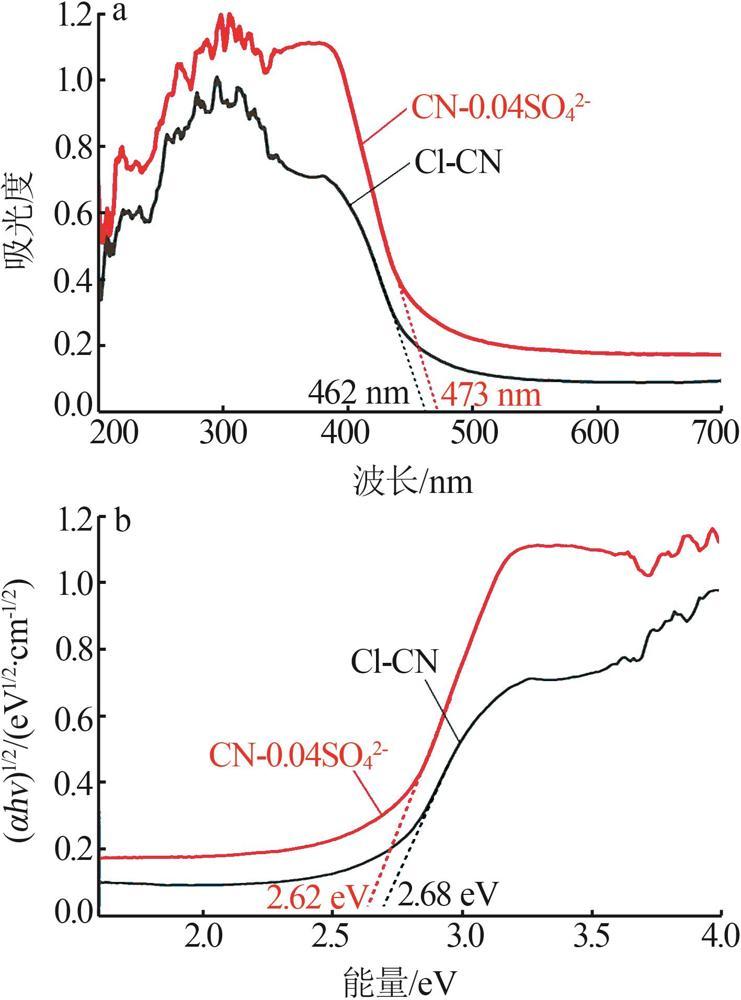
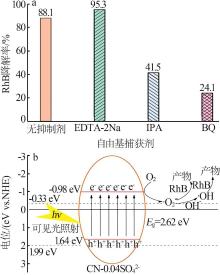
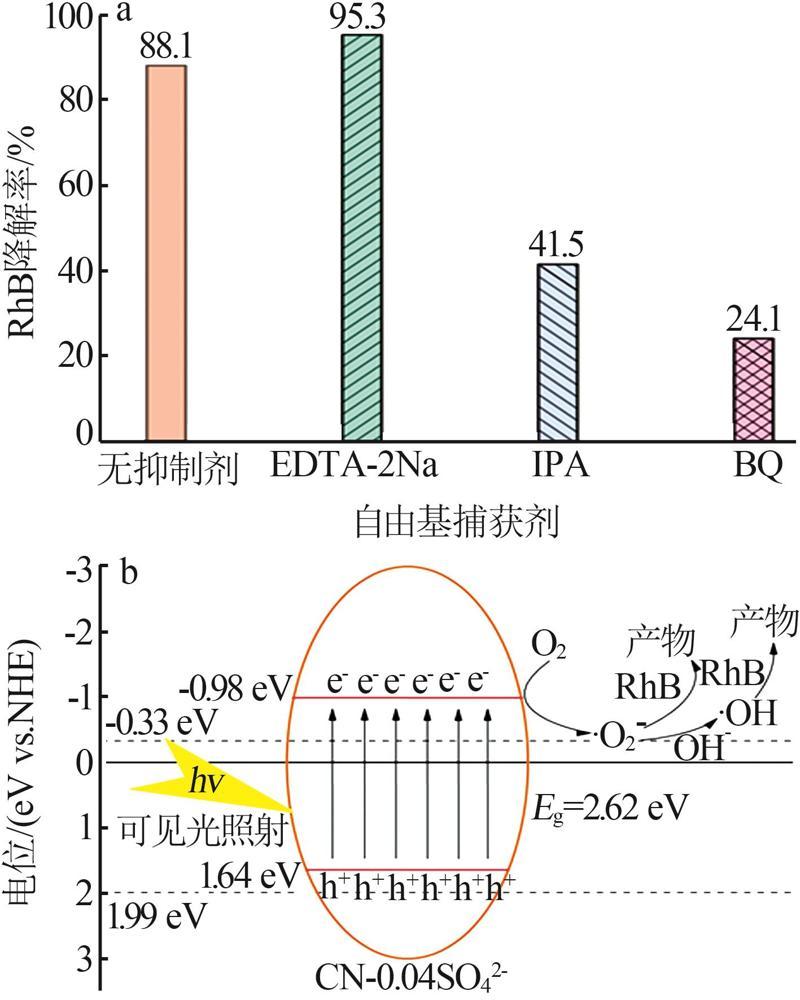
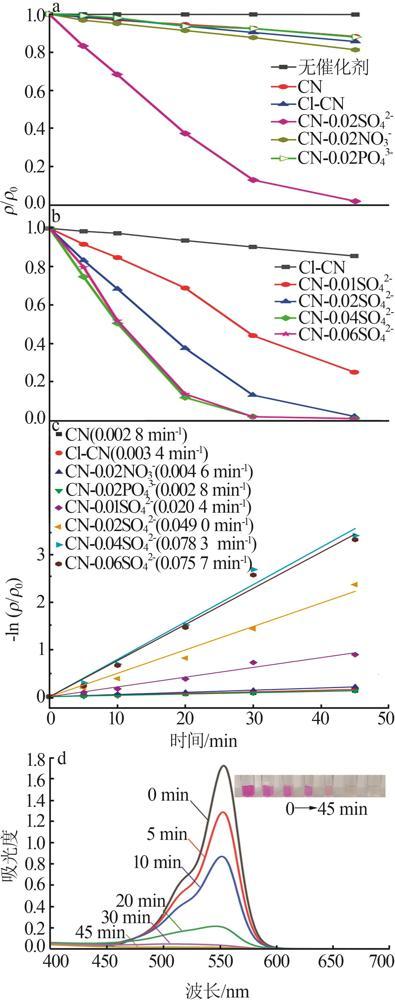
Fig.5
Photocatalytic degradation curves of RhB by catalyst?free,CN,Cl-CN,CN-0.02SO42-,CN-0.02NO3- and CN-0.02PO43-(a);The photocatalytic degradation curves of RhBby Cl-CN and CN-xSO42-(x=0.01,0.02,0.04 or 0.06)(b); Pseudo first order kinetic curves for all samples(c);UV-Vis absorption curves of RhB degradationby CN-0.04SO42-(d)"
| [1] | ONG W J, TAN L L, NG Y H, et al.Graphitic carbon nitride(g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation:Are we a step closer to achieving sustainability?[J].Chemical Reviews,2016,116(12):7159-7329. |
| [2] | PELAEZ M, NOLAN N T, PILLAI S C, et al.A review on the visible light active titanium dioxide photocatalysts for environmental applications[J].Applied Catalysis B:Environmental,2012,125:331-349. |
| [3] | WEN Jiuqing, XIE Jun, CHEN Xiaobo, et al.A review on g-C3N4-based photocatalysts[J].Applied Surface Science,2017,391:72-123. |
| [4] |
GAO Shuying, WANG Xuyu, SONG Changjian, et al.Engineering carbon-defects on ultrathin g-C3N4 allows one⁃pot output and dramatically boosts photoredox catalytic activity[J].Applied Catalysis B:Environmental,2021,295.Doi:0.1016/j.apcatb.2021.120272.
doi: 0.1016/j.apcatb.2021.120272. |
| [5] | LI Xiaogang, BI Wentuan, ZHANG Lei, et al.Single⁃atom Pt as co⁃catalyst for enhanced photocatalytic H2 evolution[J].Advanced Materials:Deerfield Beach,Fla.,2016,28(12):2427-2431. |
| [6] | FU Junwei, XU Quanlong,LOW J,et al.Ultrathin 2D/2D WO3/g-C3N4 step⁃scheme H2-production photocatalyst[J].Applied Catalysis B:Environmental,2019,243:556-565. |
| [7] | MENG Fanpeng, WANG Jun, TIAN Wenjie, et al.Graphitic carbon nitride nanosheets via acid pretreatments for promoted photocatalysis toward degradation of organic pollutants[J].Journal of Colloid and Interface Science,2022,608:1334-1347. |
| [8] | 李佳慧,李克艳,宋春山,等.聚合氮化碳的制备、改性及光催化还原二氧化碳性能研究[J].无机盐工业,2021,53(12):21-28. |
| LI Jiahui, LI Keyan, SONG Chunshan, et al.Study on preparation,modification and carbon dioxide photocatalytic reduction performance of polymeric carbon nitride[J].Inorganic Chemicals Industry,2021,53(12):21-28. | |
| [9] | WANG Longyan, HONG Yuanzhi, LIU Enli, et al.A bottom⁃up acidification strategy engineered ultrathin g-C3N4 nanosheets towards boosting photocatalytic hydrogen evolution[J].Carbon,2020,163:234-243. |
| [10] | HUANG Zhijun, LI Fengbo, CHEN Bingfeng, et al.Porous and low-defected graphitic carbon nitride nanotubes for efficient hydrogen evolution under visible light irradiation[J].RSC Advances,2015,5(124):102700-102706. |
| [11] | TANG Yunqi, YUAN Meng, JIANG Baojiang, et al.Inorganic acid-derived hydrogen⁃bonded organic frameworks to form nitro⁃gen⁃rich carbon nitrides for photocatalytic hydrogen evolution[J].Journal of Materials Chemistry A,2017,5(41):21979-21985. |
| [12] | ZHOU Chengyun, LAI Cui, HUANG Danlian, et al.Highly porous carbon nitride by supramolecular preassembly of monomers for photocatalytic removal of sulfamethazine under visible light driven[J].Applied Catalysis B:Environmental,2018,220:202-210. |
| [13] | NIU Ping, ZHANG Lili, LIU Gang, et al.Graphene⁃like carbon nitride nanosheets for improved photocatalytic activities[J].Advanced Functional Materials,2012,22(22):4763-4770. |
| [14] | LIN Qiuyan, LI Li, LIANG Shijing, et al.Efficient synthesis of monolayer carbon nitride 2D nanosheet with tunable concentration and enhanced visible⁃light photocatalytic activities[J].Applied Catalysis B:Environmental,2015,163:135-142. |
| [15] | LIU Chuang, DONG Xiaoli, HAO Yuchen, et al.A novel supramolecular preorganization route for improving g-C3N4/g-C3N4 metal⁃free homojunction photocatalysis[J].New Journal of Chemistry,2017,41(20):11872-11880. |
| [16] |
ZHOU Chengyun, ZENG Zhuotong, ZENG Guangming, et al.Visible⁃light⁃driven photocatalytic degradation of sulfamethazine by surface engineering of carbon nitride:Properties,degradation pathway and mechanisms[J].Journal of Hazardous Materials,2019,380.Doi:10.1016/j.jhazmat.2019.120815.
doi: 10.1016/j.jhazmat.2019.120815. |
| [17] | TONG Jincheng, ZHANG Li, LI Fei, et al.An efficient top-down approach for the fabrication of large⁃aspect⁃ratio g-C3N4 nano⁃sheets with enhanced photocatalytic activities[J].Physical Chemistry Chemical Physics:PCCP,2015,17(36):23532-23537. |
| [18] | CUI Yanjuan, TANG Yubin, WANG Xinchen.Template⁃free synthesis of graphitic carbon nitride hollow spheres for photocatalytic degradation of organic pollutants[J].Materials Letters,2015, 161:197-200. |
| [19] | CUI Yanjuan, DING Zhengxin, FU Xianzhi, et al.Construction of conjugated carbon nitride nanoarchitectures in solution at low temperatures for photoredox catalysis[J].Angewandte Chemie:International Ed.in English,2012,51(47):11814-11818. |
| [20] |
LI Xibao, KANG Bangbang, DONG Fan, et al.Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies[J].Nano Energy,2021,81.Doi:10.1016/j.nanoen.2020.105671.
doi: 10.1016/j.nanoen.2020.105671. |
| [21] | LAN Huachun, LI Lili, AN Xiaoqiang, et al.Microstructure of carbon nitride affecting synergetic photocatalytic activity:Hydrogen bonds vs.structural defects[J].Applied Catalysis B:Environmental,2017,204:49-57. |
| [22] | YU Kai, YANG Shaogui, HE Huan, et al.Visible light-driven photocatalytic degradation of rhodamine B over NaBiO3:Pathways and mechanism[J].The Journal of Physical Chemistry A,2009,113(37):10024-10032. |
| [23] | HU Shaozheng, MA Lin, YOU Jiguang, et al.Enhanced visible light photocatalytic performance of g-C3N4 photocatalysts co⁃do⁃ped with iron and phosphorus[J].Applied Surface Science,2014, 311:164-171. |
| [24] | 班昌胜,李军,金央,等.超分子前体制备g-C3N4/g-C3N4同质结及光催化性能研究[J].无机盐工业,2022,54(3):125-131. |
| BAN Changsheng, LI Jun, JIN Yang, et al.Study on preparation of g-C3N4/g-C3N4 homojunction by supramolecular precursor and its photocatalytic property[J].Inorganic Chemicals Industry,2022,54(3):125-131. |
| [1] | YAO Jiankang, HU Shuozhen, NIU Dongfang, WU Jianping, ZHANG Xinsheng. Study on electrochemical treatment of sodium chloride organic waste salt in spice industry [J]. Inorganic Chemicals Industry, 2024, 56(3): 105-115. |
| [2] | HUANG Jianan, LU Xiaoyu, WANG Mitang. Effect of Ba-La co-doping on degradation of methylene blue dye by TaON [J]. Inorganic Chemicals Industry, 2024, 56(2): 146-151. |
| [3] | ZHANG Lijie, LI Degang, HAN Wenyuan, XU Huijun, ZHANG Weimin, YU Chen. Preparation of phosphorus-doped carbon quantum dots and activation of peroxymonosulfate for degradation of methylene blue [J]. Inorganic Chemicals Industry, 2024, 56(1): 126-133. |
| [4] | ZHAO Yan, HAO Xuewei, SHI Hainan, LI Jiahui, LI Keyan, GUO Xinwen. Study on photocatalytic CO2 reduction performance of Cu-doped TiO2/PCN heterojunction [J]. Inorganic Chemicals Industry, 2023, 55(8): 21-27. |
| [5] | YAN Chaoqun, ZHANG Xianming, WEI Juan, CHENG Zhiliang, XU Qian, ZHANG Xuan. Synthesis of cubic α-Fe2O3 catalyst and its photo-Fenton degradation performance of antibiotic under visible light [J]. Inorganic Chemicals Industry, 2023, 55(8): 28-35. |
| [6] | PANG Fei, XU Yingrui, CHAI Chunling, SHEN Jingjing, BAI Liguang, ZHAO Xiaodong. Overview on recycling of waste activated alumina in production of hydrogen peroxide by anthraquinone process [J]. Inorganic Chemicals Industry, 2023, 55(6): 1-7. |
| [7] | LAN Yinghua, CHEN Yanmei, MA Ruixiao, ZHANG Yanhui. Preparation and photocatalytic performance of Ce-Ti oxide-attapulgite composites [J]. Inorganic Chemicals Industry, 2023, 55(4): 133-140. |
| [8] | LI Hongyuan,HARI Bala. Research progress of preparation and photocatalytic application of metal halide perovskite quantum dots [J]. Inorganic Chemicals Industry, 2023, 55(2): 36-44. |
| [9] | TONG Haijuan,LI Siqi,FAN Fangfang,ZUO Weiyuan,SHI Bingfang. Facile synthesis of bismuth oxychloride and its photocatalytic degradation performance of p-nitrophenol [J]. Inorganic Chemicals Industry, 2022, 54(9): 157-162. |
| [10] | SONG Zhi, LIU Boxia, CHEN Yaoyao. Study on synthesis of FeWO4/WO3 complex by sol-gel and degradation of textile dye wastewater [J]. Inorganic Chemicals Industry, 2022, 54(5): 131-137. |
| [11] | JI Chang,WANG Guosheng. Degradation of berberine by visible light over Ag3PO4 /g-C3N4 heterojunction catalyst [J]. Inorganic Chemicals Industry, 2022, 54(4): 175-180. |
| [12] | WANG Xin,XUE Dongfeng. Preparation of oxynitrides photocatalysts by the oriented transformation strategy [J]. Inorganic Chemicals Industry, 2022, 54(3): 1-6. |
| [13] | LI Xiangyang,LIU Ziwei,LI Keyan,GUO Xinwen. Study on preparation and performance of FeMn-MOF/CN heterojunction photo-Fenton catalyst [J]. Inorganic Chemicals Industry, 2022, 54(12): 126-132. |
| [14] | LI Jiahui,WANG Huan,LI Keyan,GUO Xinwen. Study on photocatalytic CO2 reduction performance of Co doped polymeric carbon nitride [J]. Inorganic Chemicals Industry, 2022, 54(11): 124-130. |
| [15] | WANG Xiaohuan,LI Shenghao,SHI Zhiming,WANG Jun,XINBA Yaer,LIU Liang. Research status of FeTiO3 materials [J]. Inorganic Chemicals Industry, 2022, 54(1): 12-17. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||