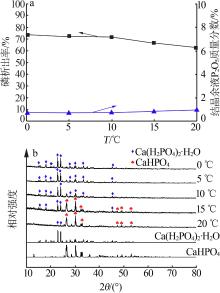
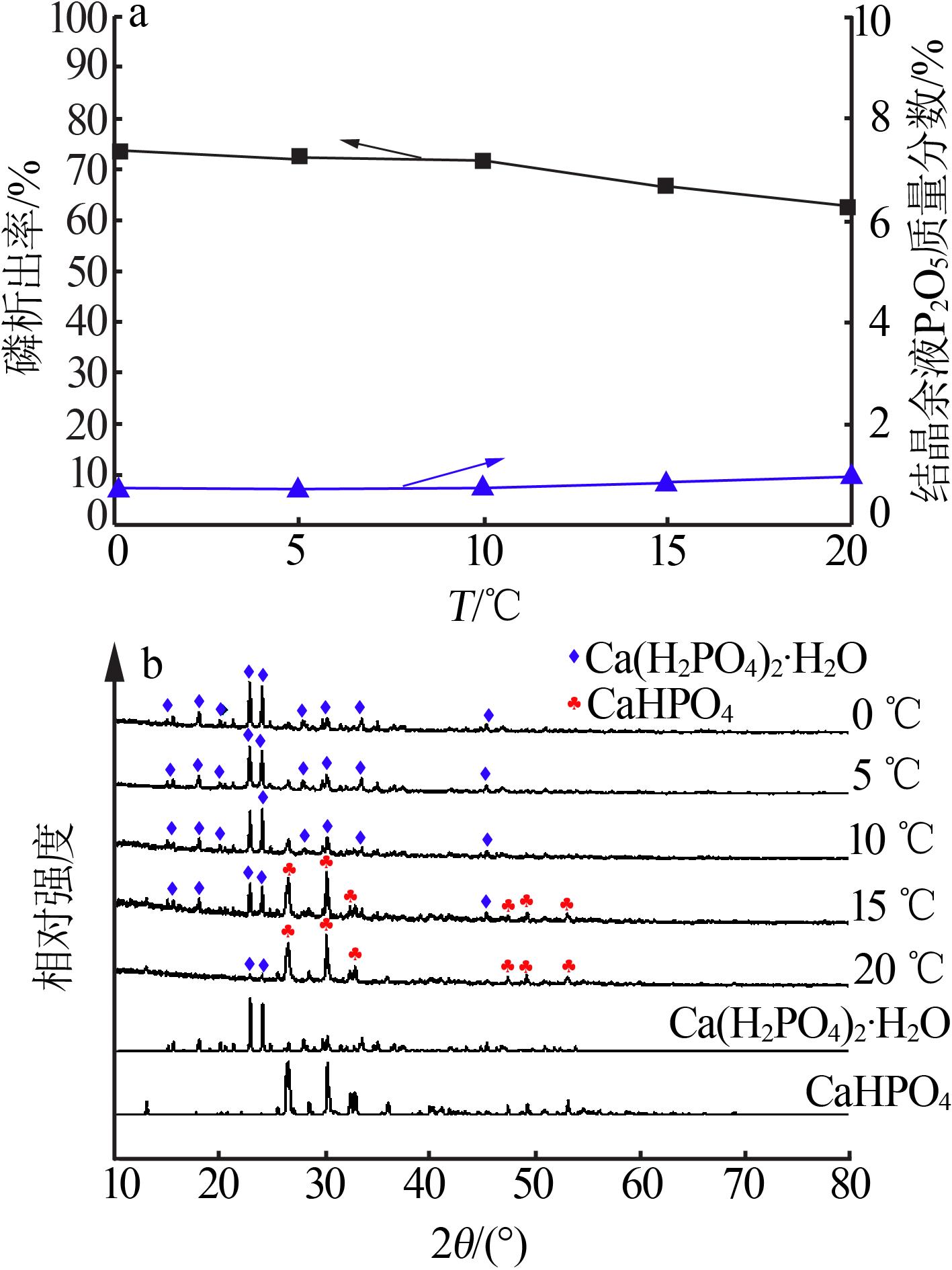
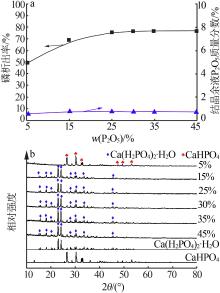
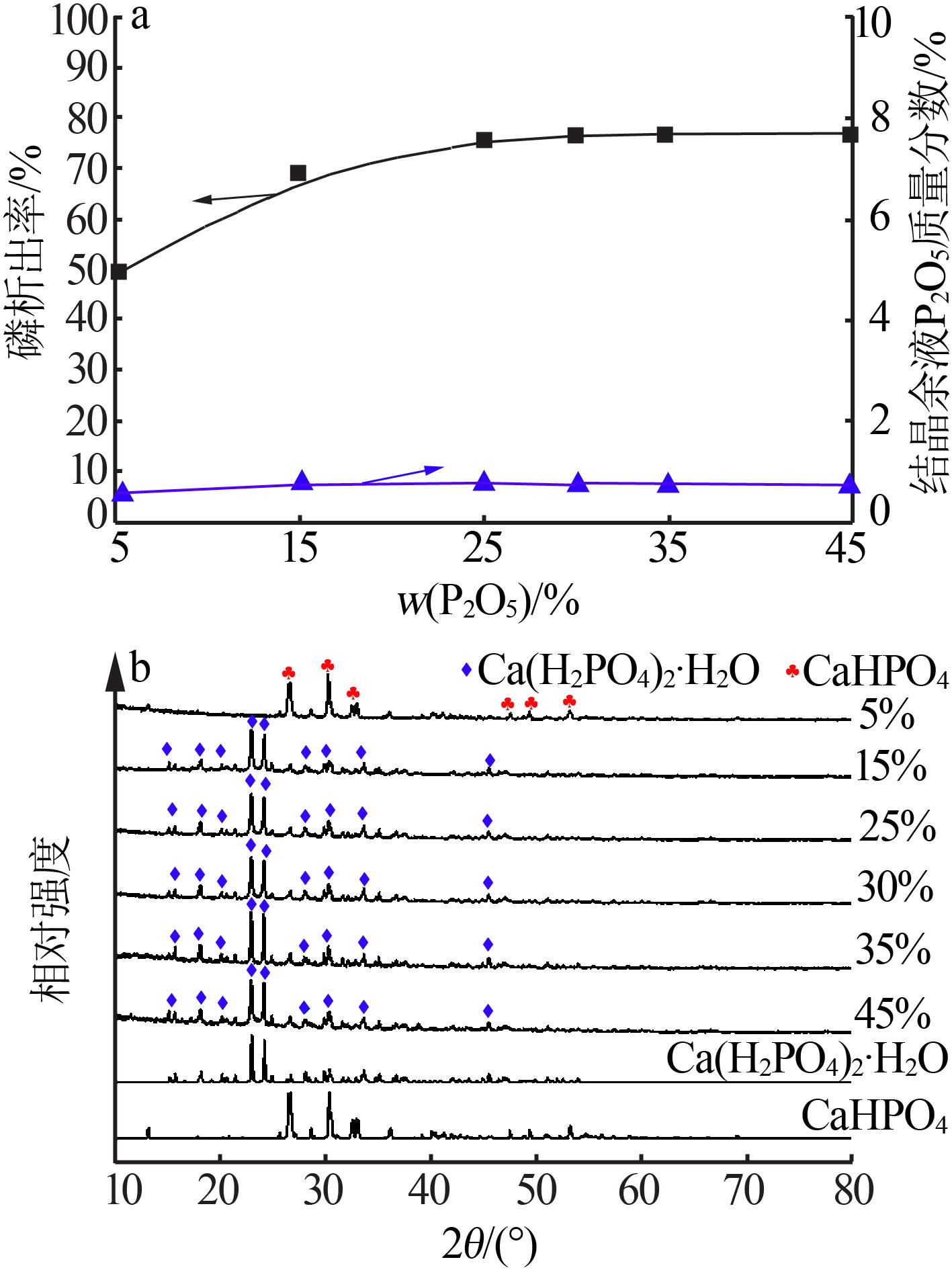
| 1 |
白海丹.2019年我国磷石膏利用现状、问题及建议[J].硫酸工业,2020(12):7-10.
|
|
BAI Haidan.Current situation,problems and suggestions of phosphogypsum utilization of 2019 in China[J].Sulphuric Acid Industry,2020(12):7-10.
|
| 2 |
吴照洋,张永兴,张利珍,等. β石膏相组成和杂质含量对其性能的影响[J].无机盐工业,2021,53(9):67-71.
|
|
WU Zhaoyang, ZHANG Yongxing, ZHANG Lizhen,et al.Effect of phase composition and impurity content of β-gypsum on its properties[J].Inorganic Chemicals Industry,2021,53(9):67-71.
|
| 3 |
顾春光.硝酸分解磷矿综合利用设想[J].硫磷设计与粉体工程,2013(1):18-23,49.
|
|
GU Chunguang.Idea for comprehensive utilization of phosphorite with nitric acid decomposition[J].Sulphur Phosphorus & Bulk Materials Handling Related Engineering,2013(1):18-23,49.
|
| 4 |
孙志岩.磷矿-硝酸分解液的冷冻结晶实验研究[J].磷肥与复肥,2012,27(2):13-14,17.
|
|
SUN Zhiyan.Research on freezing crystallization of phosphate decomposed liquid by nitric acid[J].Phosphate & Compound Fertilizer,2012,27(2):13-14,17.
|
| 5 |
吴德桥,陈红琼,钟本和,等.我国发展硝酸磷肥的生产工艺探讨[J].磷肥与复肥,2009,24(4):36-39.
|
|
WU Deqiao, CHEN Hongqiong, ZHONG Benhe,et al.Discussion on process for nitrophosphate production in China[J].Phosphate & Compound Fertilizer,2009,24(4):36-39.
|
| 6 |
李朝荣,苏殊,杨秀山,等.硝酸法湿法磷酸工艺的研究进展[C]∥2020中国环境科学学会科学技术年会.南京:中国环境科学学会,2020.
|
|
LI Chaorong, SU Shu, YANG Xiushan,et al.Research progress of nitric acid wet method for phosphoric acid process[C]∥2020 CSES Annual Conference on Environmental Science and Technology.Nanjing:CSES,2020.
|
| 7 |
ALFASSI Z B, MOSSERI S.Solventing out of electrolytes from their aqueous solution[J].AIChE Journal,1984,30(5):874-876.
|
| 8 |
ZHOU Xiaohou, ZHENG Wenjia, XU Dehua,et al.Solubility measurement and thermodynamics modelling for potassium dihydrogen phosphate in a water-ethanol system from 293.2 to 323.2 K[J].Fluid Phase Equilibria,2020,512.Doi:org/10.1016/j.fluid.2020 .
doi: org/10.1016/j.fluid.2020
|
|
112533.
doi: org/10.1016/j.fluid.2020
|
| 9 |
鲍颖,王永莉,王静康.溶析结晶研究进展[J].化学工业与工程,2004,21(6):438-443.
|
|
BAO Ying, WANG Yongli, WANG Jingkang.Progress in dilution crystallization[J].Chemical Industry and Engineering,2004,21(6):438-443.
|
| 10 |
李朝荣,苏殊,许德华,等.磷酸二氢钙在硝酸钙-磷酸-水溶液体系中溶析结晶工艺[J].高校化学工程学报,2021,35(4):608-615.
|
|
LI Chaorong, SU Shu, XU Dehua,et al.Antisolvent crystallization of monocalcium phosphate in calcium nitratephosphoric acid-water solution system[J].Journal of Chemical Engineering of Chinese Universities,2021,35(4):608-615.
|
| 11 |
张焕焕,刘艺,张兰,等.无水磷酸氢钙固化姜黄素磷脂复合物[J].中成药,2020,42(1):20-24.
|
|
ZHANG Huanhuan, LIU Yi, ZHANG Lan,et al.Solidification of curcumin phospholipid complex with anhydrous calcium hydrogen phosphate[J].Chinese Traditional Patent Medicine,2020,42(1):20-24.
|
| 12 |
李云东,马航,刘晨曦.牙膏用二水磷酸氢钙生产工艺的改进探索[J].现代化工,2021,41(9):232-234,240.
|
|
LI Yundong, MA Hang, LIU Chenxi.Research in improving process for production of toothpaste-purposed calcium hydrogen phosphate dihydrate[J].Modern Chemical Industry,2021,41(9):232-234,240.
|
| 13 |
李漱阳,李鸿,王鹏,等.可降解磷酸钙生物陶瓷的制备与性能[J].功能材料,2015,46(24):24147-24152.
|
|
LI Shuyang, LI Hong, WANG Peng,et al.The preparation and properties of degradable calcium phosphate bioceramics[J].Journal of Functional Materials,2015,46(24):24147-24152.
|
| 14 |
LEI Shuoyi, ZHAO Yuming, HAN Jianmin.Effect of monocalcium phosphate on the physical,chemical,mechanical and biological properties of calcium silicate cement:An in vitrostudy[J].Advances in Applied Ceramics,2020,119(5/6):348-356.
|
| 15 |
LIU Qingying, YE Huaqun, XU Minglei,et al.The effect and mechanism of dietary monocalcium phosphate restriction on hepatic lipid deposition and immune status in obscure puffer,Takifugu obscurus[J].Aquaculture,2020,524.Doi:org/10.1016/j.aquaculture.2020.735261 .
doi: org/10.1016/j.aquaculture.2020.735261
|
| 16 |
王和庆,乐清华,徐菊美,等.溶析结晶法脱除钛白废酸中硫酸亚铁盐的研究[J].高校化学工程学报,2015,29(3):697-702.
|
|
WANG Heqing, LE Qinghua, XU Jumei,et al.Removal of ferrous sulfate from waste acids of titanium dioxide industry by solvent-
|
|
ing-out crystallization[J].Journal of Chemical Engineering of
|
|
Universities Chinese,2015,29(3):697-702.
|
| 17 |
BOONCHOM B.Parallelogram-like microparticles of calcium dihydrogen phosphate monohydrate(Ca(H2PO4)2·H2O) obtained by a rapid precipitation route in aqueous and acetone media[J].Journal of Alloys and Compounds,2009,482(1/2):199-202.
|
| 18 |
黄炎,孙海龙,孟子超,等.溶析结晶在医药领域的研究进展[J].化工进展,2019,38(5):2380-2388.
|
|
HUANG Yan, SUN Hailong, MENG Zichao,et al.Progress in antisolvent crystallization in pharmaceutical field[J].Chemical Industry and Engineering Progress,2019,38(5):2380-2388.
|
| 19 |
曹维文,李军,陈明,等.磷酸氢钙结晶聚结过程及主要影响杂质的探究[J].无机盐工业,2021,53(1):50-53.
|
|
CAO Weiwen, LI Jun, CHEN Ming,et al.Study on agglomeration process during crystallization of calcium hydrogen phosphate and its main affecting impurity thereof[J].Inorganic Chemicals Industry,2021,53(1):50-53.
|
 ),WANG Bo,XU Dehua(
),WANG Bo,XU Dehua( ),YANG Xiushan,ZHANG Zhiye(
),YANG Xiushan,ZHANG Zhiye( )
)