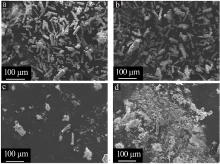
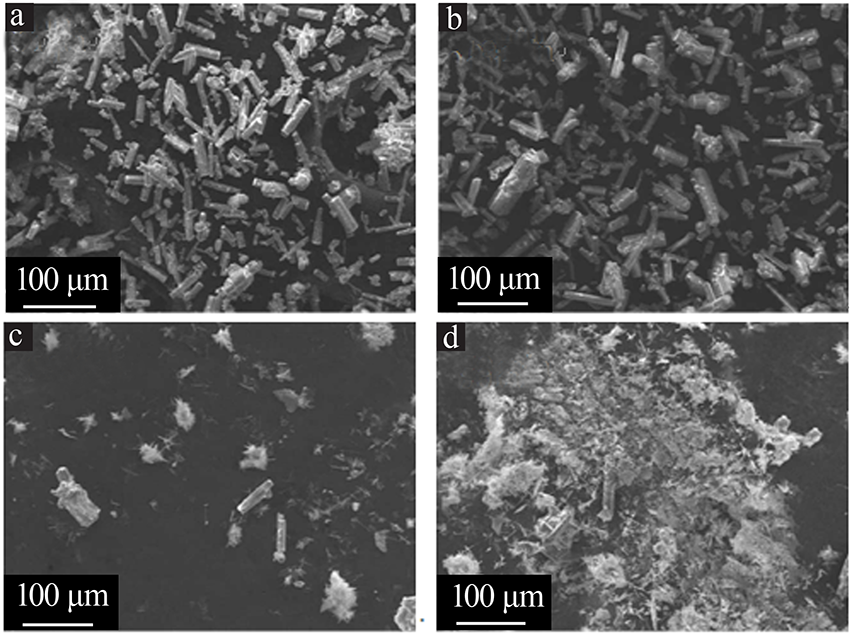
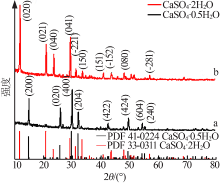
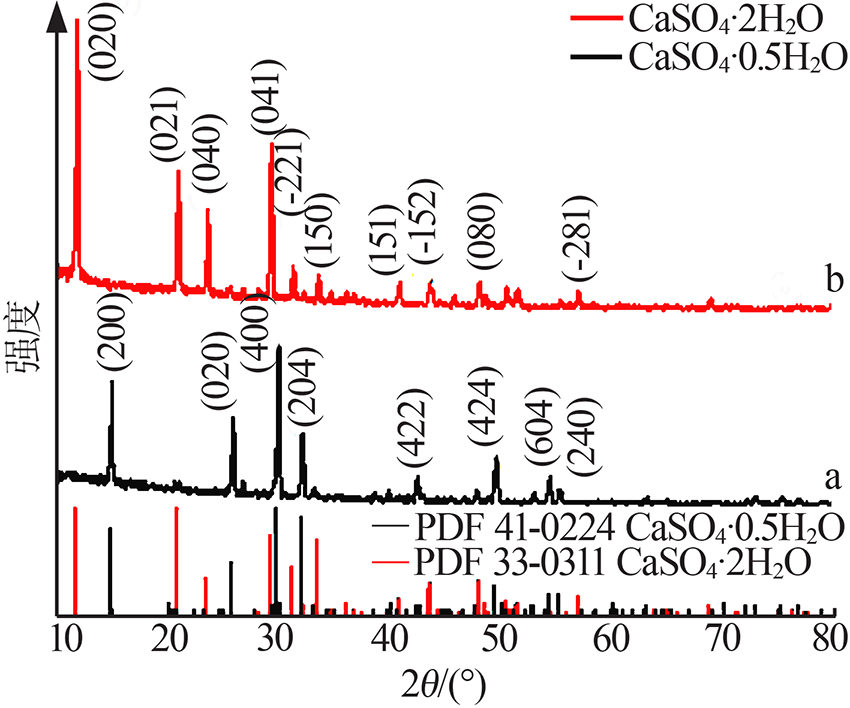
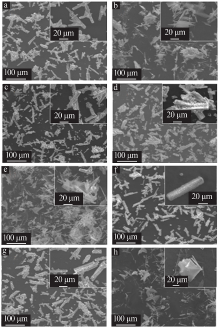
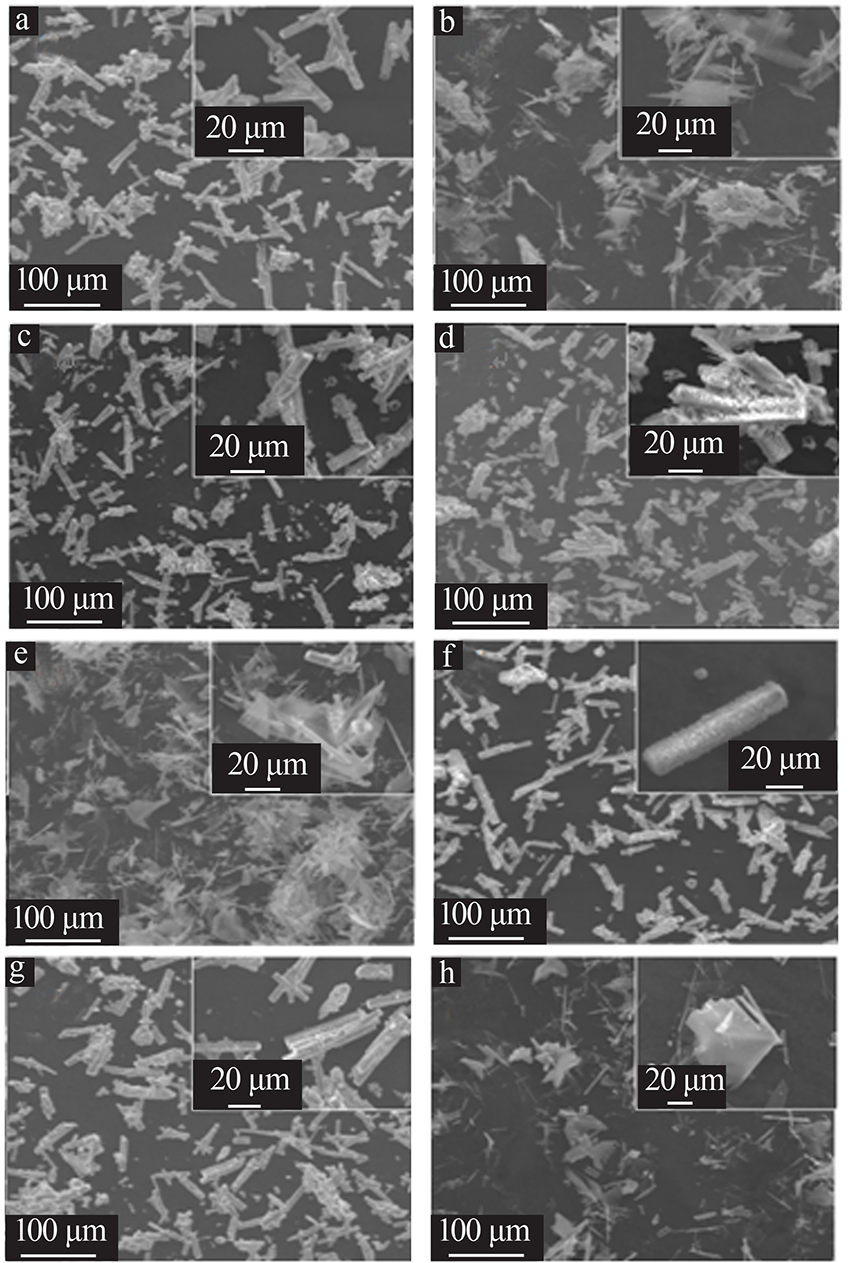
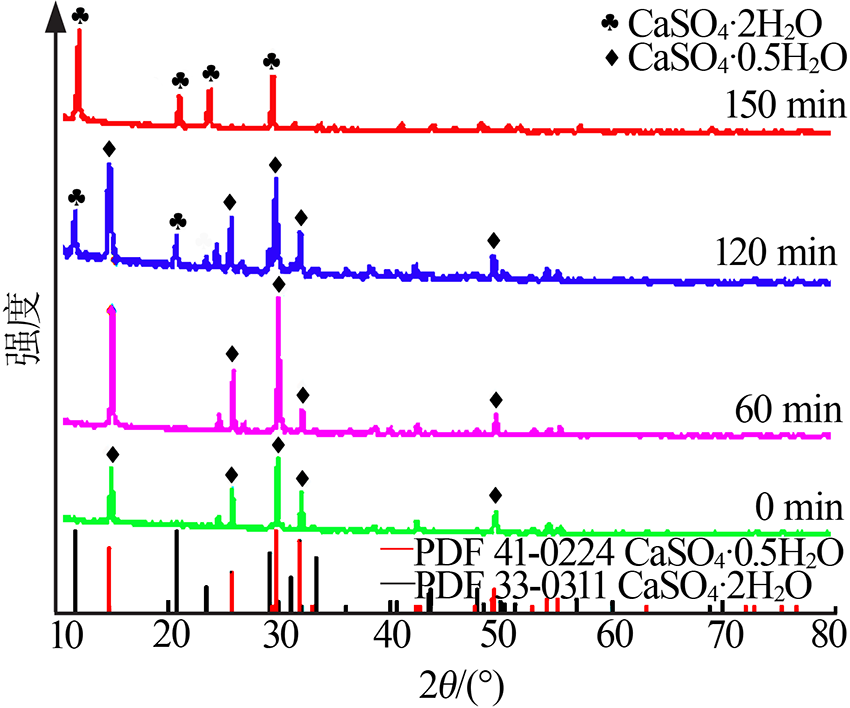
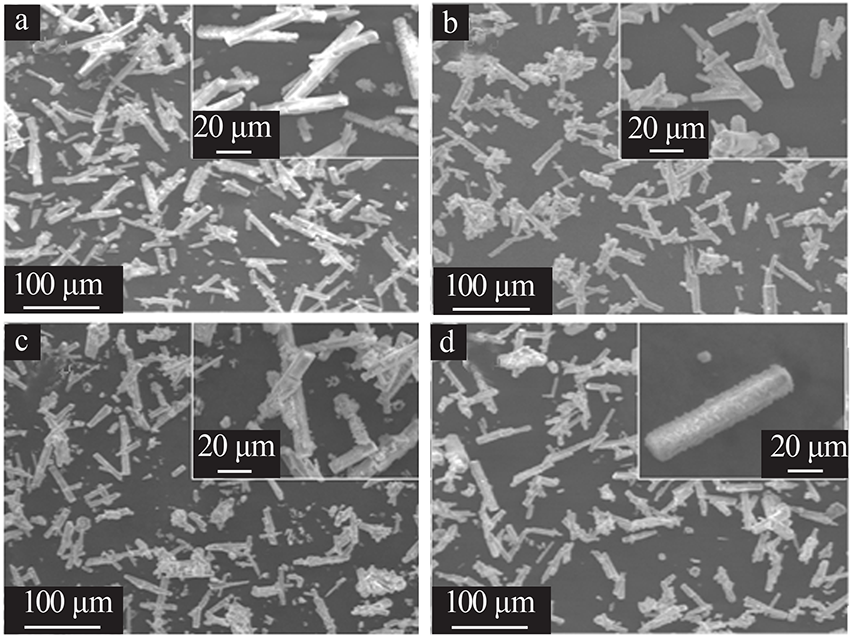
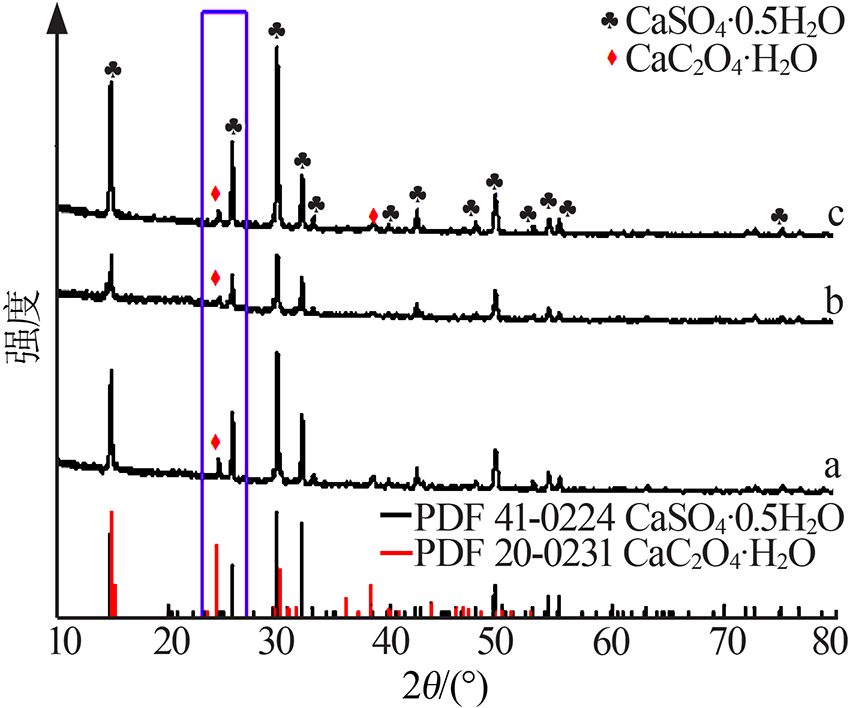
| [1] |
BAO G M, WEN D L, YING S, et al. Synjournal of α-hemihydrate gypsum from cleaner phosphogypsum[J]. Journal of Cleaner Produc-tion, 2018, 195:396-405.
|
| [2] |
QING J G, YUE H H, HONG H T, et al. Preparation of α-CaSO4 · 1/2H2O with tunable morphology from flue gas desulphurization gyp-sum using malic acid as modifier:A theoretical and experimental study[J]. Journal of Colloid and Interface Science, 2018, 530:292-301.
doi: 10.1016/j.jcis.2018.06.068
|
| [3] |
XIAN B L, QIN Z, BAO L K, et al. Insight into the effect of maleic acid on the preparation of α-hemihydrate gypsum from phospho-gypsum in Na2SO4 solution[J]. Journal of Crystal Growth, 2018, 493:34-40.
doi: 10.1016/j.jcrysgro.2018.04.025
|
| [4] |
张凡凡, 陈超, 张西兴, 等. 不同来源石膏的性能特点与应用分析[J]. 无机盐工业, 2017, 49(8):10-13.
|
| [5] |
王宇斌, 张鲁, 毛欣钰, 等. 氢氧根对半水硫酸钙晶体形貌的影响机理[J]. 无机盐工业, 2020, 52(11):20-23,55.
|
| [6] |
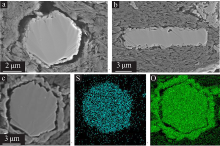
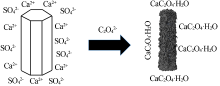
高传慧, 董亚洁, 任秀, 等. 半水硫酸钙晶须的水化机理及稳定性调节[J]. 无机化学学报, 2020, 36(5):142-154.
|
| [7] |
羊道和, 杨玉荣, 易美桂. 柠檬酸对半水硫酸钙晶须形貌稳定性影响[J]. 人工晶体学报, 2019, 48(1):95-100.
|
| [8] |
成诚, 陈德玉, 王舒州, 等. 硅酸钠湿法改性CaSO4·1/2H2O晶须及其作用机理[J]. 西南科技大学学报, 2017, 32(4):7-12.
|
| [9] |
刘红叶, 刘富玲, 王宇斌, 等. 磷酸钠对半水硫酸钙晶须的稳定作用[J]. 化工矿物与加工, 2011, 40(4):11-13.
|
| [10] |
FREYER D, VOIGT W . Crystallization and phase stability of CaSO4 and CaSO4 -based salts[J]. Monatshefte Für Chemie, 2003, 134(5):693-719.
doi: 10.1007/s00706-003-0590-3
|
| [11] |
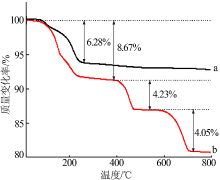
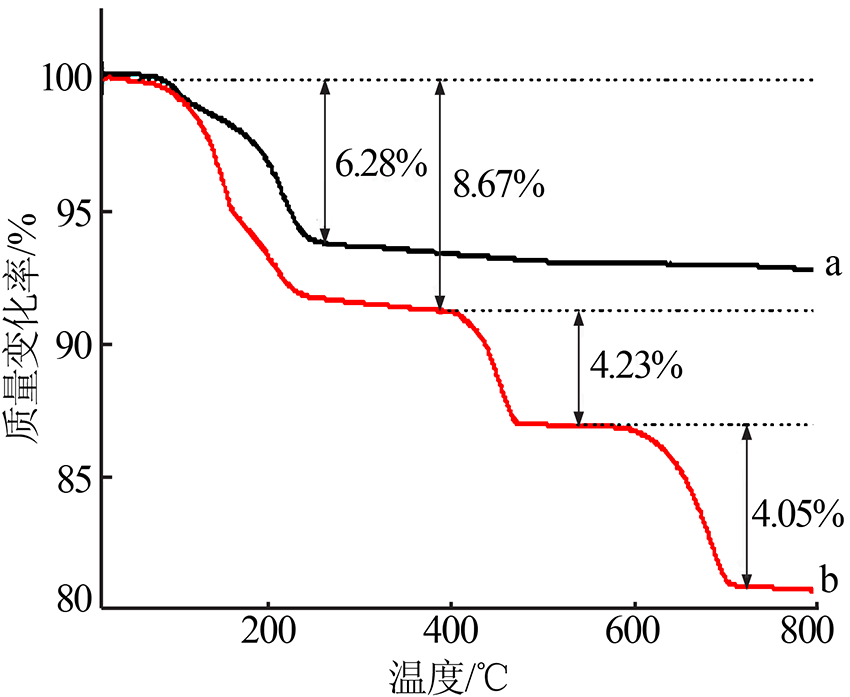
章斐. 用TG/FTIR/MS联用技术研究草酸钙分解过程中CO和CO2的共逸出[J]. 分析仪器, 2017(6):108-116.
|
| [12] |
陈畅, 史思涛, 伍勇华, 等. 常温改性半水硫酸钙晶须及其稳定化机理研究[J]. 人工晶体学报, 2018, 47(4):32-39.
|
 ),HU Meng1,DOU Yan1(
),HU Meng1,DOU Yan1( ),CUI Peng1,SHEN Hao2,ZHENG Zhiyin2,LIU Rong2
),CUI Peng1,SHEN Hao2,ZHENG Zhiyin2,LIU Rong2