Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (7): 83-85.doi: 10.19964/j.issn.1006-4990.2020-0511
• Industrial Techniques • Previous Articles Next Articles
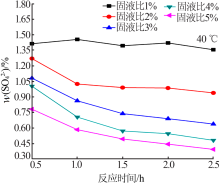
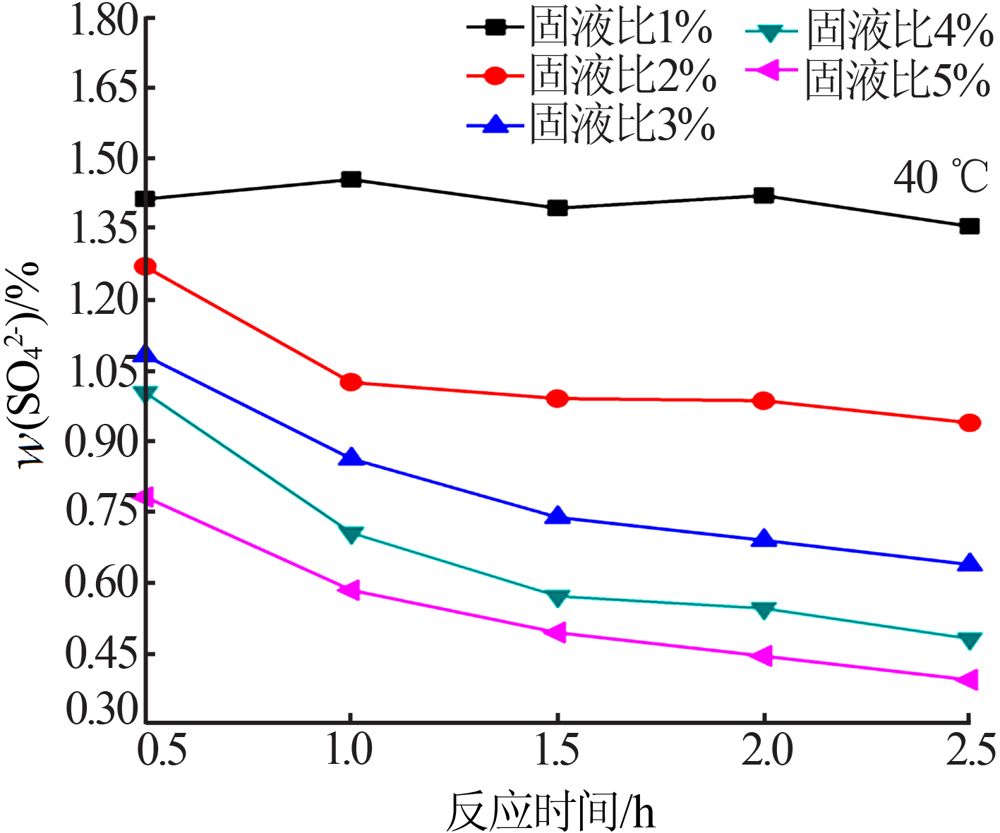
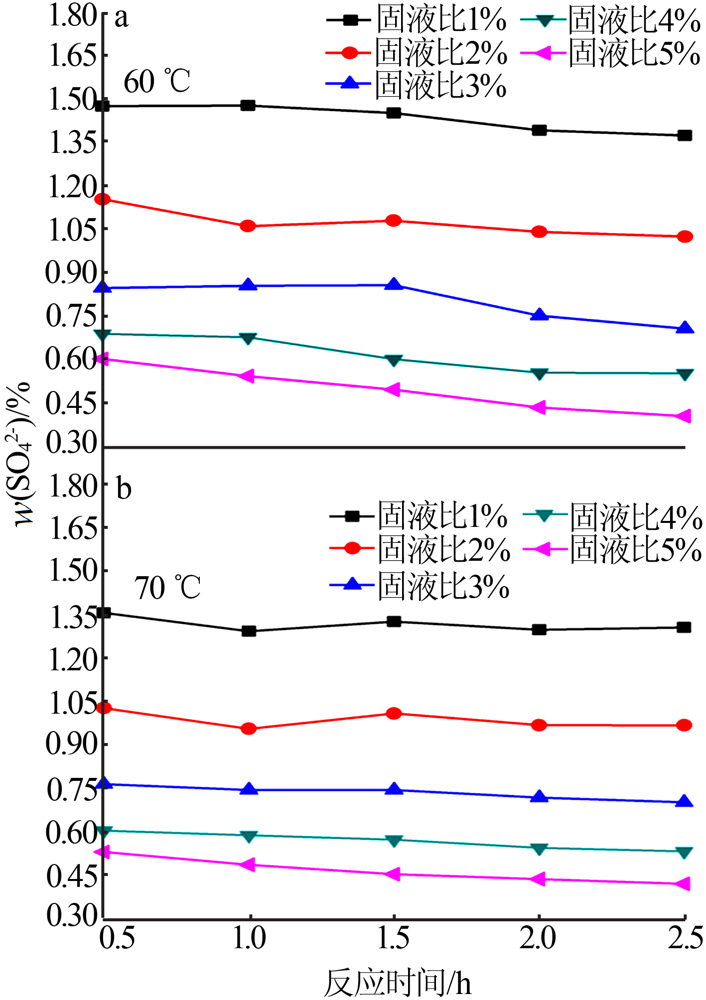
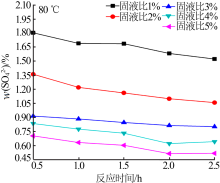
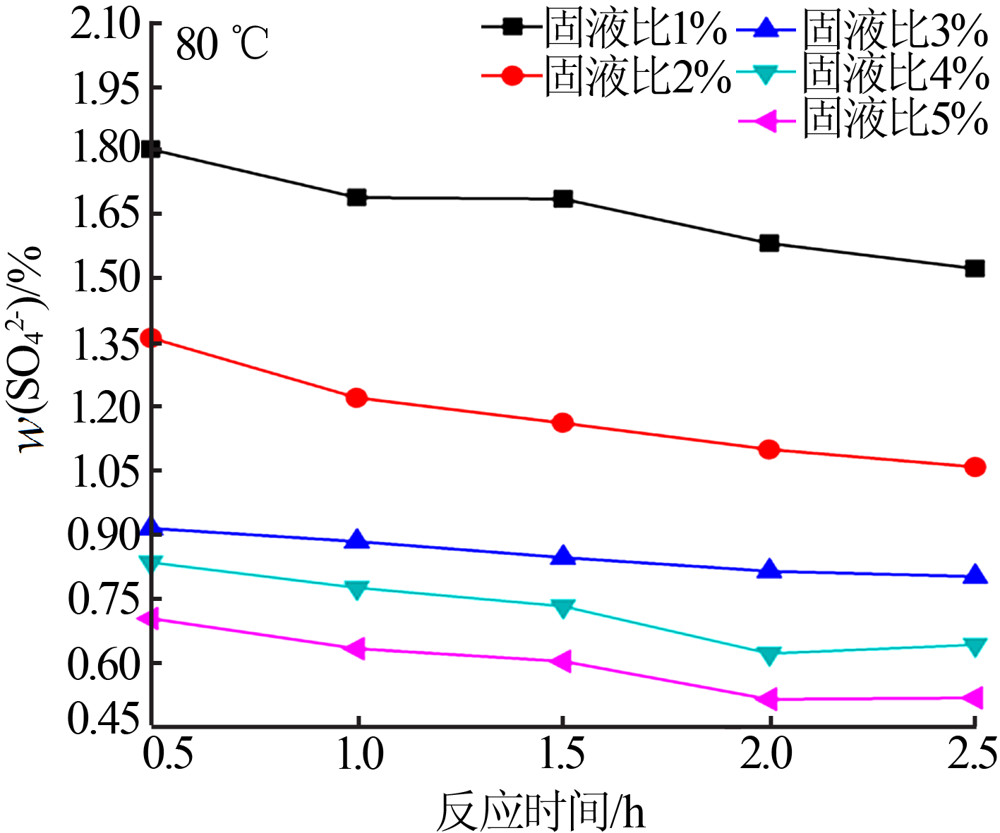
Experimental research on sulfate removal from wet-process phosphoric acid using phosphate concentrate
Han Zenghui1( ),Zhou Qiongbo2,Xie Jianxin1,Zeng Yanping1,Cui Yunchun1,Lin Hong1
),Zhou Qiongbo2,Xie Jianxin1,Zeng Yanping1,Cui Yunchun1,Lin Hong1
- 1. School of Chemistry Biology and Environment,Yuxi Normal University,Yuxi 653100,China
2. Yunnan Phosphate Chemical Group Co.Ltd.