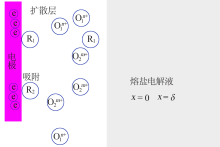
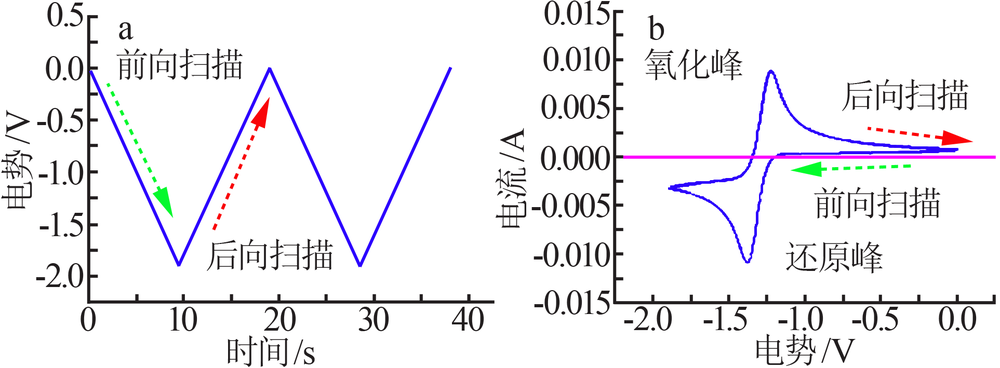
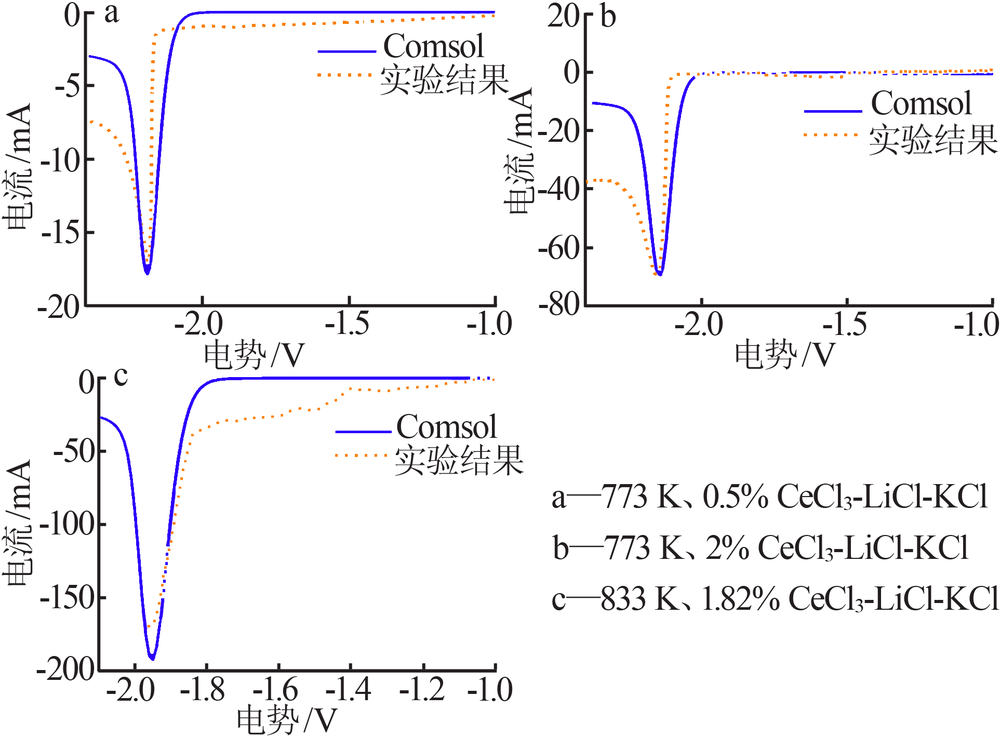
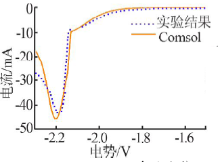
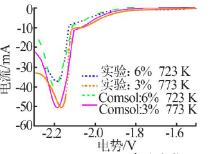
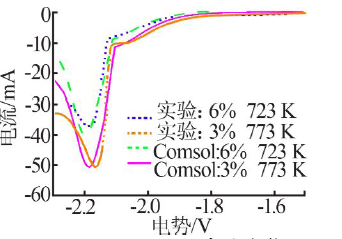
| [1] |
王有群, 何辉, 林如山, 等. 无机氯化物熔盐在乏燃料干法后处理中的应用进展[J]. 无机盐工业, 2016,48(8):1-5.
|
| [2] |
黄一飞, 孟照凯, 林如山, 等. 氯化锂-氯化钾熔盐中二氧化铈的熔解还原[J]. 无机盐工业, 2016,48(10):16-19.
|
| [3] |
张萌, 王靖阳, 孙兰昕, 等. 乏燃料干法后处理中铀电沉积行为模拟研究[J]. 核动力工程, 2019,40(6):72-76.
|
| [4] |
Kim S H, Park S B, Lee S, et al. Computer-assisted design and experi-mental validation of multielectrode electrorefiner for spent nuclear fuel treatment using a tertiary model[J]. Nuclear Engineering and Design, 2013,257:12-20.
|
| [5] |
Zhou W, Wang Y, Zhang J. Integrated model development for safe-guarding pyroprocessing facility:Part Ⅰ-Model development and va-lidation[J]. Annals of Nuclear Energy, 2018,112:603-614.
doi: 10.1016/j.anucene.2017.09.050
|
| [6] |
Zhou W, Wang Y, Zhang J. Integrated model development for safe-guarding pyroprocessing facility:Part Ⅱ-Case studies and model integration[J]. Annals of Nuclear Energy, 2018,112:48-61.
doi: 10.1016/j.anucene.2017.09.036
|
| [7] |
Zhang M, Wang J, Cai Y, et al. Electroanalytical and electrodeposit-ed simulation of Ce3+ in molten LiCl-KCl [J]. Journal of the Elec-trochemical Society, 2019,166(15):D868-D874.
|
| [8] |
Zhang J. Safeguards in pyroprocessing:An integrated model develop-ment and measurement data analysis[D]. Columbus,OH:The Ohio State University Research Foundation, 2017.
|
| [9] |
Berzins T, Delahay P. Oscillographic polarographic waves for the re-versible deposition of metals on solid electrodes[J]. Journal of the American Chemical Society, 1953,75(3):555-559.
|
| [10] |
Delahay P. New instrumental methods in electrochemistry[J]. In-strumentation and Application to Analytical and Physical Chem-istry,Interscience, 1954,102(2):46C-47C.
|
| [11] |
朱凤艳. Ce(Ⅲ)在熔盐中的电化学行为及共沉积制备Mg-Li-Ce、Al-Li-Ce合金[D]. 哈尔滨:哈尔滨工程大学, 2012.
|
| [12] |
Wu E. Integrated study of rare earth drawdown by electrolysis for mo-lten salt recycle[D].Columbus,Ohio:The Ohio State University, 2017
|
 ),Wang Dezhong(
),Wang Dezhong( )
)