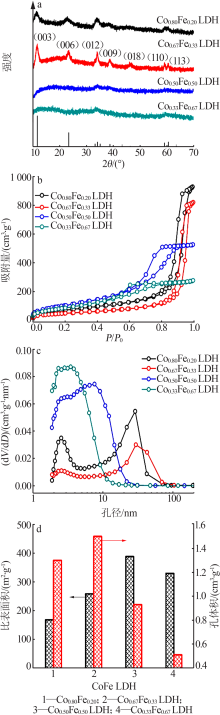
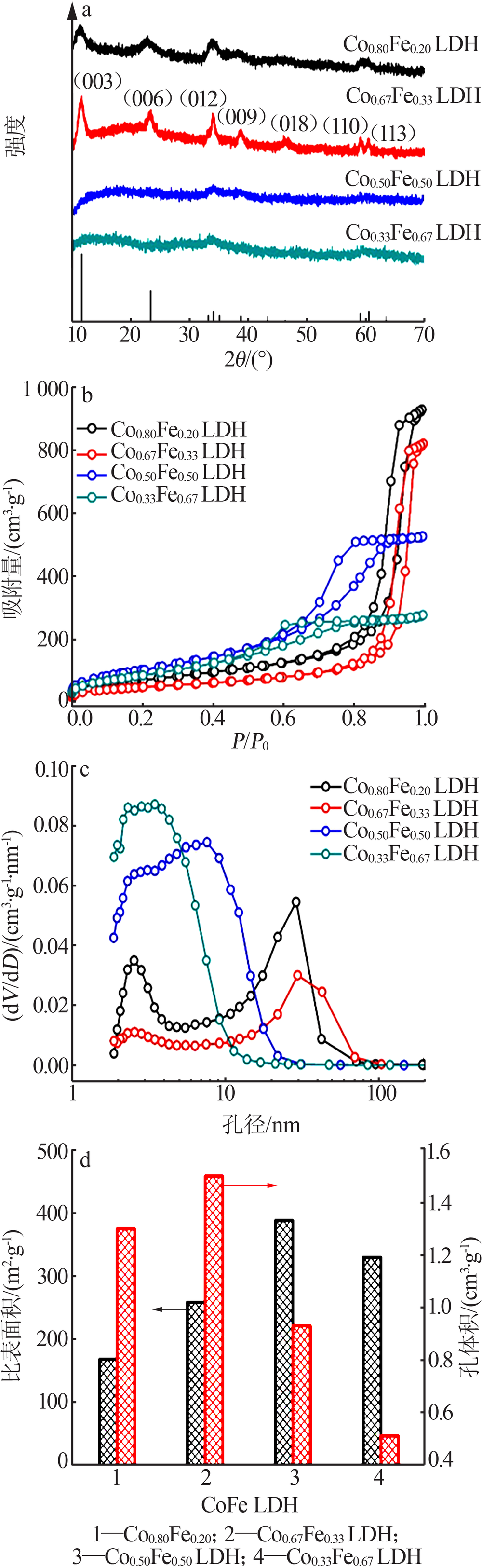
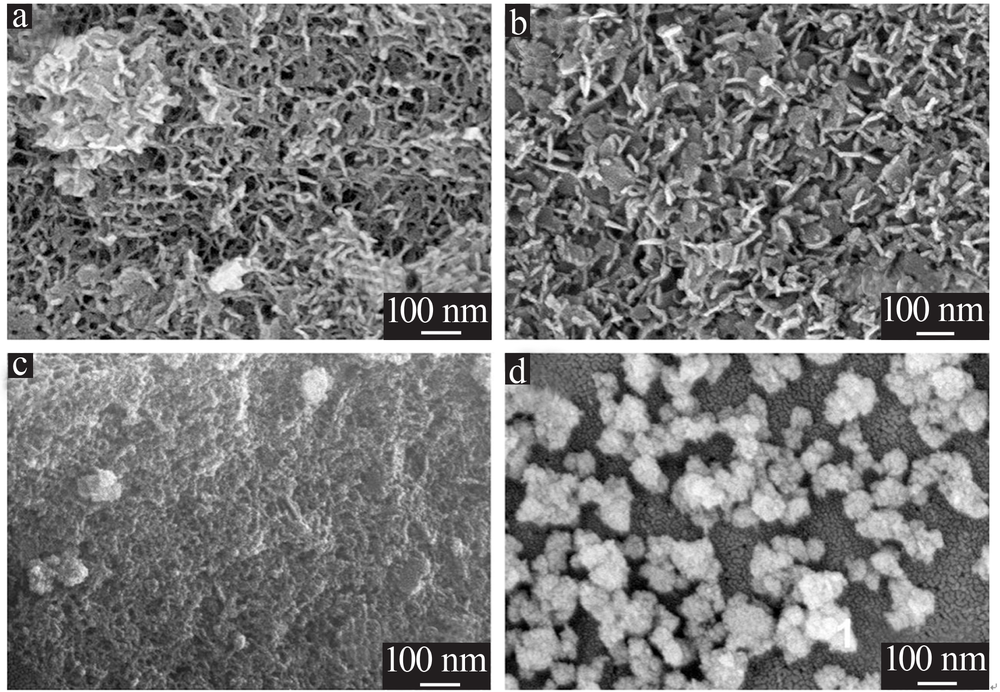
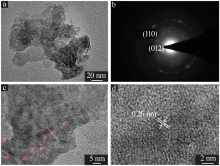
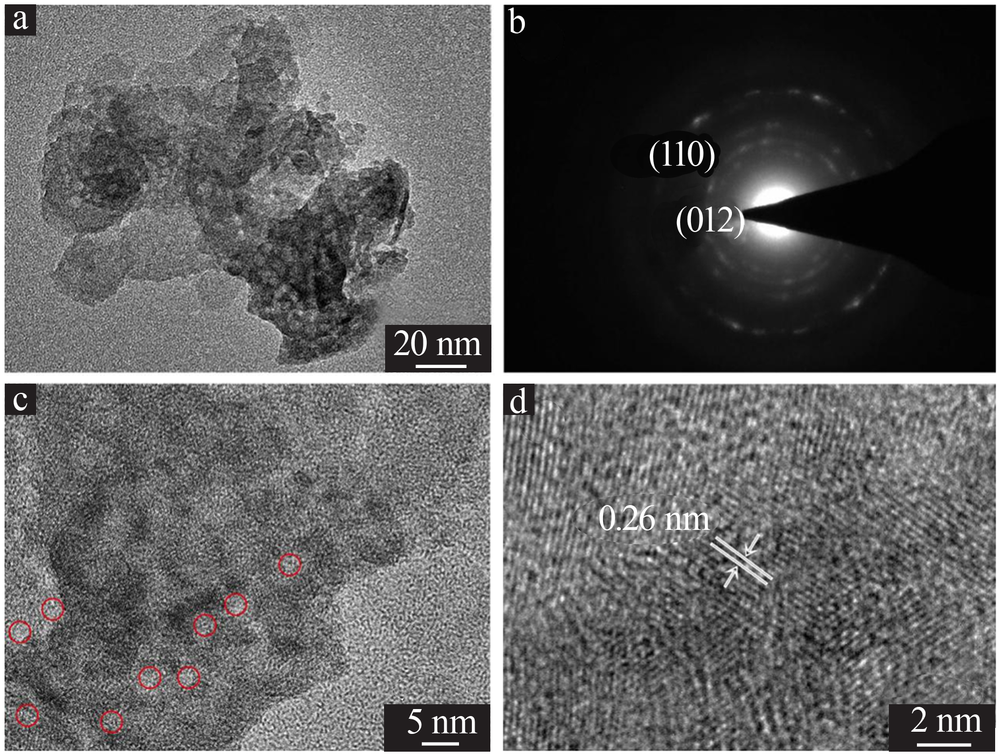
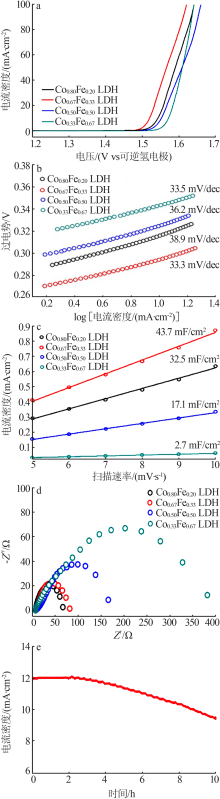
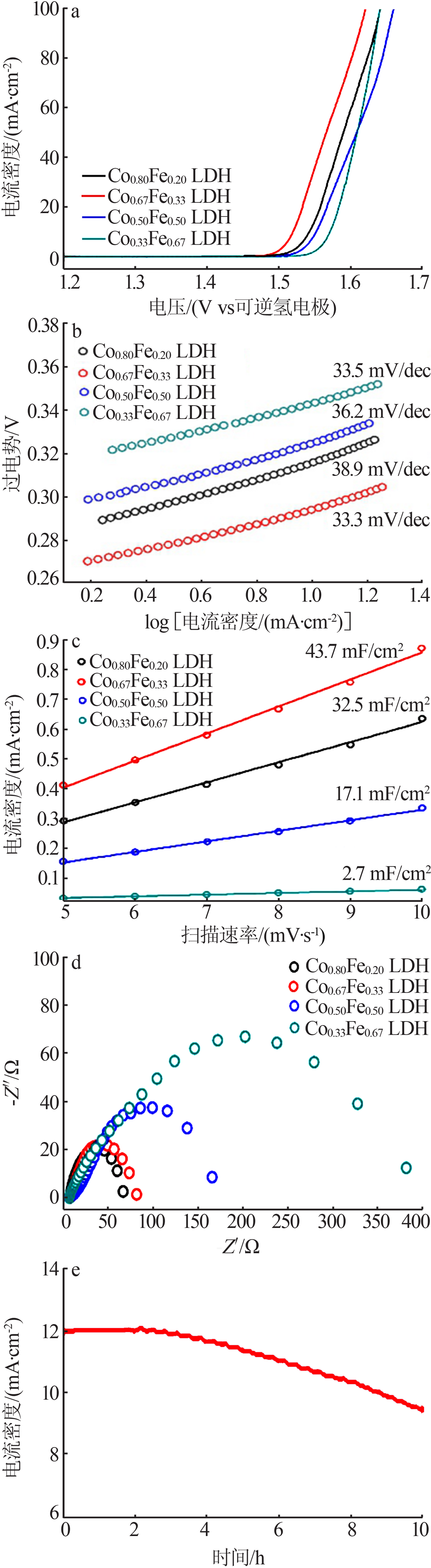
| [1] |
Mahmood N, Yao Y, Zhang J, et al. Electrocatalysts for hydrogen evolution in alkaline electrolytes:Mechanisms,challenges,and prospective solutions[J]. Advanced Science, 2018,5(2):1700464.
doi: 10.1002/advs.201700464
pmid: 29610722
|
| [2] |
何杨华, 徐金铭, 王发楠, 等. Ni-Fe基析氧阳极材料的研究进展[J]. 化工进展, 2016,35(7):2057-2062.
|
| [3] |
Jamesh M, Sun X. Recent progress on earth abundant electrocatalysts for oxygen evolution reaction(OER) in alkaline medium to achieve efficient water splitting-A review[J]. Journal of Power Sources, 2018,400:31-68.
|
| [4] |
王雅, 方志强, 史晓雨, 等. 镍铁双金属系列电催化材料的研究进展[J]. 化学研究, 2018,29(6):638-641.
|
| [5] |
赵宇, 张硕嘉, 徐冰, 等. 锌基复合电极原位水热合成和电化学性能研究[J]. 无机盐工业, 2019,51(6):21-24.
|
| [6] |
谢博尧, 张纪梅, 郝帅帅, 等. 层状双氢氧化物析氧催化剂的研究进展[J]. 材料工程, 2020,48(1):1-9.
|
| [7] |
Huang L, Zou Y, Chen D, et al. Electronic structure regulation on layered double hydroxides for oxygen evolution reaction[J]. Chinese Journal of Catalysis, 2019,40(12):1822-1840.
doi: 10.1016/S1872-2067(19)63284-5
|
| [8] |
任锦, 梁良, 周瑜, 等. 功能化层状双金属氢氧化物材料的应用进展[J]. 材料科学与工程学报, 2019,37(3):509-516.
|
| [9] |
Cai Z, Bu X, Wang P, et al. Recent advances in layered double hydroxide electrocatalysts for the oxygen evolution reaction[J]. Journal of Materials Chemistry A, 2019,7(10):5069-5089.
doi: 10.1039/C8TA11273H
|
| [10] |
Yin H, Tang Z. Ultrathin two-dimensional layered metal hydroxides:an emerging platform for advanced catalysis,energy conversion and storage[J]. Chemical Society Reviews, 2016,45(18):4873-4891.
doi: 10.1039/c6cs00343e
pmid: 27373467
|
| [11] |
Yang Y, Ou Y, Yang Y, et al. Modulated transition metal-oxygen covalency in the octahedral sites of CoFe layered double hydroxides with vanadium doping leading to highly efficient electrocatalysts[J]. Nanoscale, 2019,11(48):23296-23303.
doi: 10.1039/c9nr08795h
pmid: 31782483
|
| [12] |
Li J, Li X, Luo Y, et al. Cobalt carbonate hydroxide mesostructure with high surface area for enhanced electrocatalytic oxygen evolution[J]. International Journal of Hydrogen Energy, 2018,43(20):9635-9643.
doi: 10.1016/j.ijhydene.2018.03.229
|
| [13] |
Yang Y, Dang L, Shearer M, et al. Highly active trimetallic NiFeCr layered double hydroxide electrocatalysts for oxygen evolution reaction[J]. Advanced Energy Materials, 2018,8(15):1703189.
doi: 10.1002/aenm.v8.15
|
| [14] |
Cai Z, Zhou D, Wang M, et al. Introducing Fe2+ into nickel-iron alyered double hydroxide:local structure modulated water oxidation activity[J]. Angewandte Chemie International Edition, 2018,57(30):9392-9396.
doi: 10.1002/anie.201804881
pmid: 29889350
|
| [15] |
Yang T, Ye Q, Liang Y, et al. Graded holey nickel cobalt layered double hydroxide nanosheet array electrode with high mass loading for high-energy-density all-solid-state supercapacitors[J]. Journal of Power Sources, 2020,449:227590.
doi: 10.1016/j.jpowsour.2019.227590
|
| [16] |
麦诗欣, 程高, 余林, 等. 碳纸负载钴氧化物的制备及电催化析氧性能研究[J]. 无机盐工业, 2020,52(1):87-92.
|
 ),Xu Xuetang(
),Xu Xuetang( ),Wang Fan
),Wang Fan