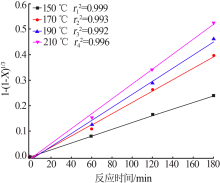
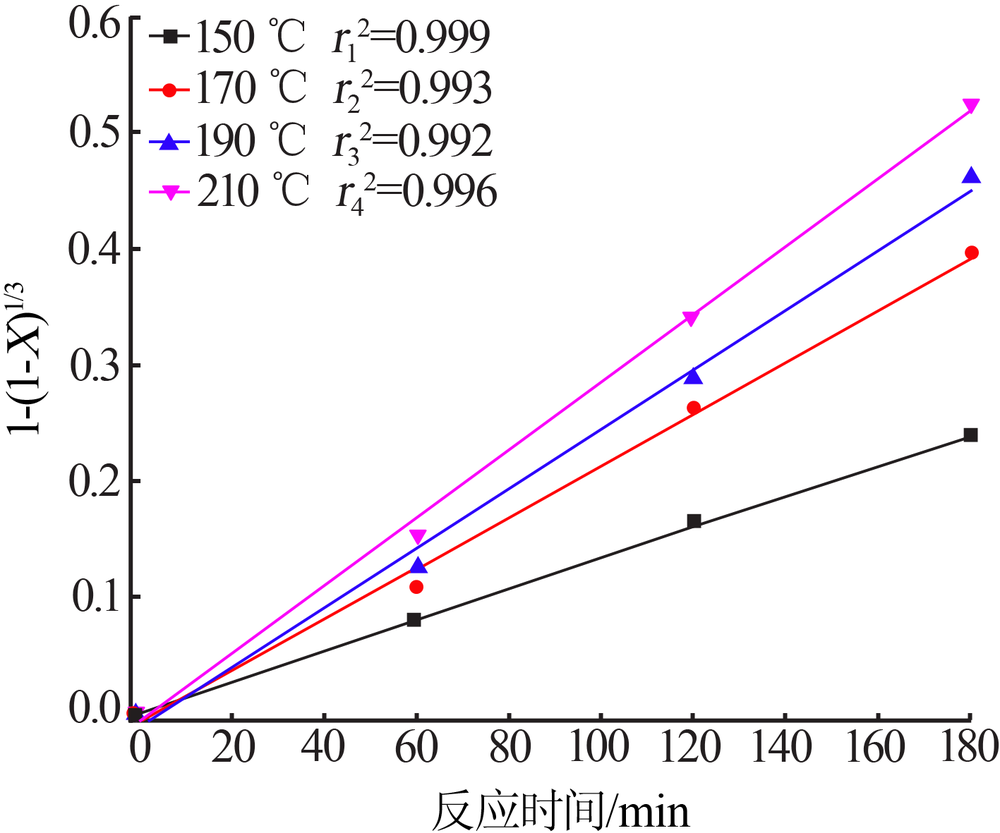
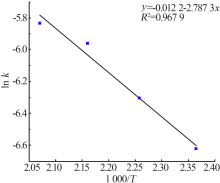
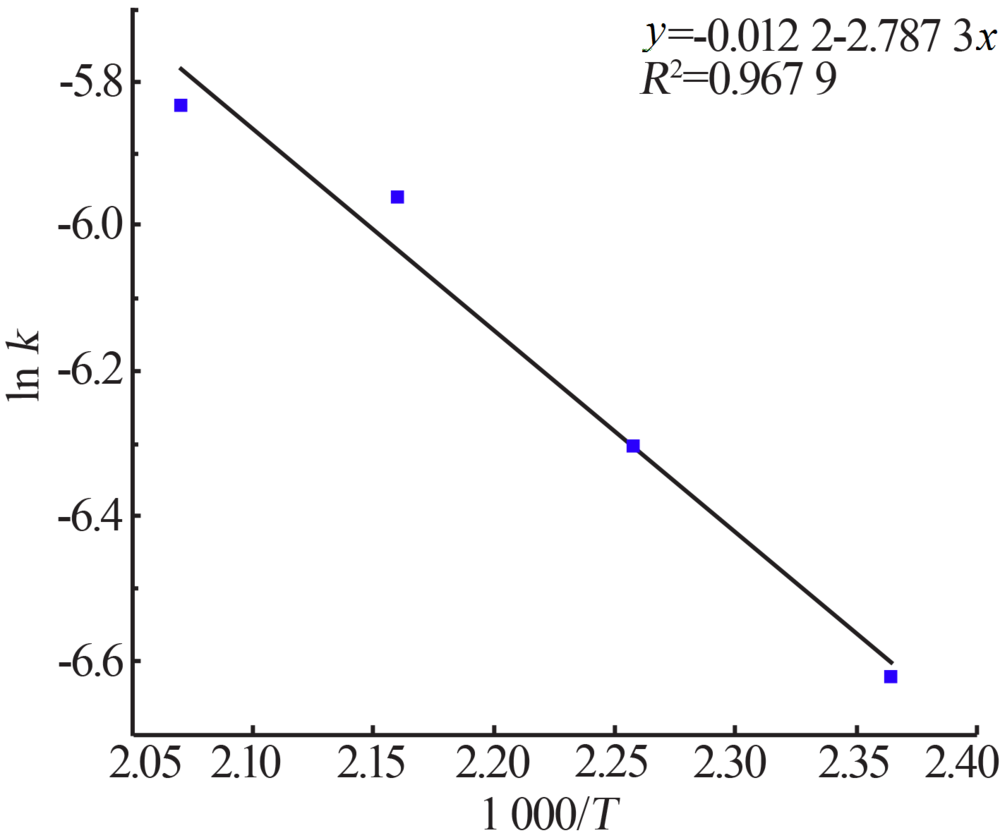
Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (11): 86-90.doi: 10.11962/1006-4990.2019-0637
• Environment·Health·Safety • Previous Articles Next Articles
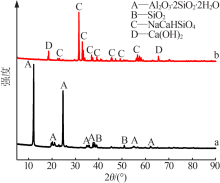
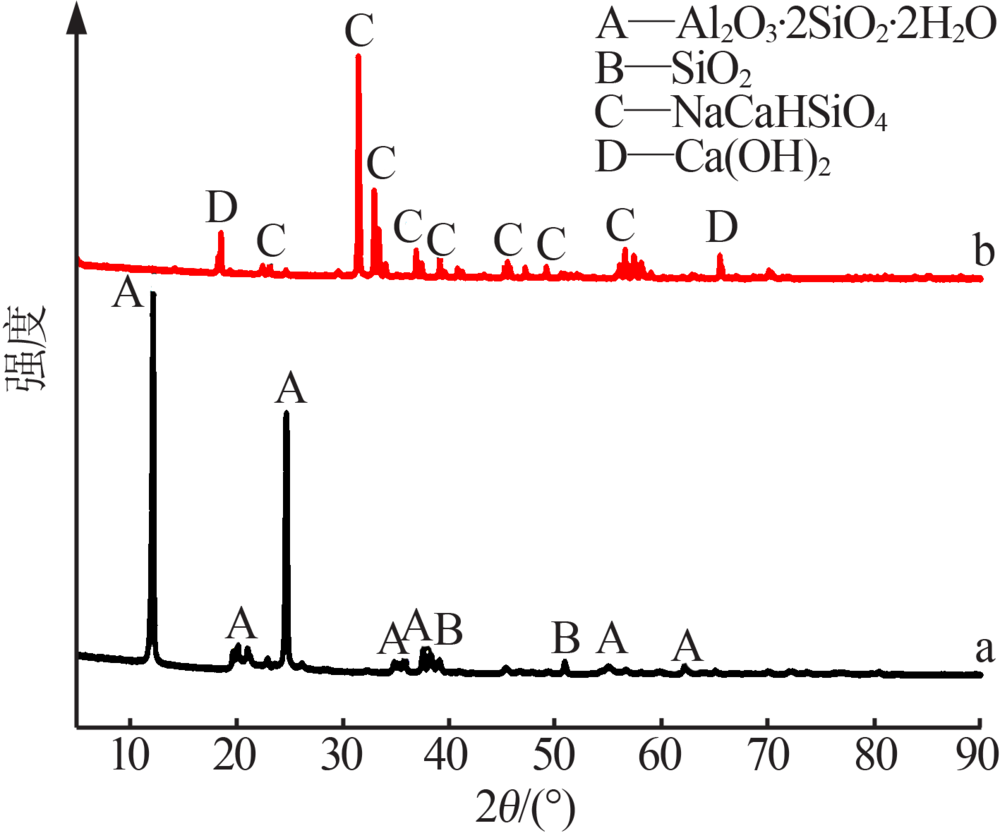
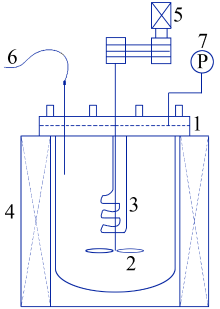
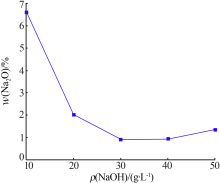
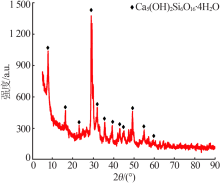
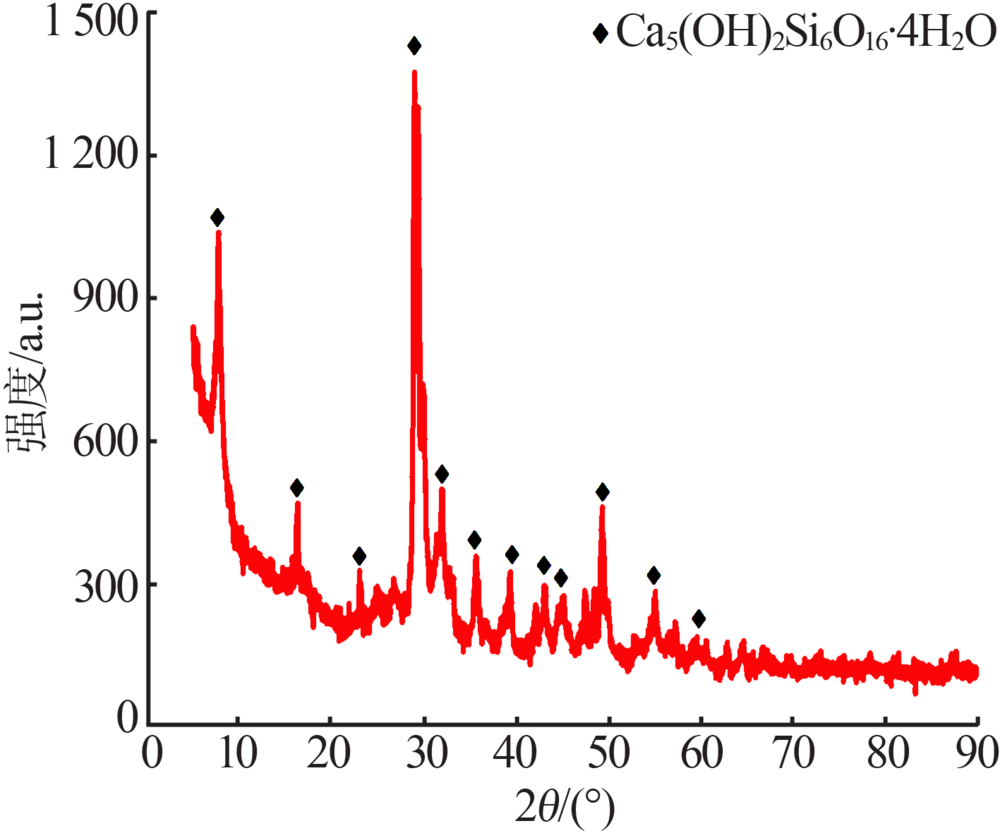
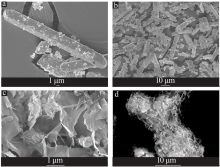
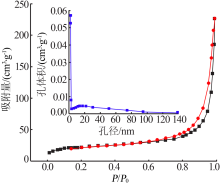
Preparation of mesoporous calcium silicate with alumina-extracted coal gangue by hydrothermal method
Yang Quancheng1,2( ),Gong Zhiming3,Mao Yanyu4,Li Xiaodong1,Zhang Yancheng1,Shi Changsheng1,Zeng Ming2
),Gong Zhiming3,Mao Yanyu4,Li Xiaodong1,Zhang Yancheng1,Shi Changsheng1,Zeng Ming2
- 1. Department of Environmental Engineering,North China Institute of Science and Technology,Beijing 101601,China
2. School of Chemical and Environmental Engineering,China University of Mining and Technology(Beijing)
3. Yangquan No.3 Coal Mine Preparation Plant,Yangquan Coal Industry(Group) Co.,Ltd.
4. Clean Coal Company Jin Hua Gong Coal Preparation Plant,Datong Coal Mine Group Co.,Ltd.
-
Received:2020-05-13Online:2020-11-10Published:2020-12-01
CLC Number:
Cite this article
Yang Quancheng,Gong Zhiming,Mao Yanyu,Li Xiaodong,Zhang Yancheng,Shi Changsheng,Zeng Ming. Preparation of mesoporous calcium silicate with alumina-extracted coal gangue by hydrothermal method[J]. Inorganic Chemicals Industry, 2020, 52(11): 86-90.
share this article
| [1] | 郭彦霞, 张圆圆, 程芳琴. 煤矸石综合利用的产业化及其展望[J]. 化工学报, 2014,65(7):2443-2453. |
| [2] | 石松林, 刘钦甫, 孙俊民, 等. 准格尔煤田高铝煤矸石中勃姆石富集特征及成因[J]. 煤炭工程, 2014,46(5):116-118,122. |
| [3] | 滕英跃, 张永锋, 白杰, 等. 高铝煤矸石制备超细氧化铝和硅酸钠联产工艺[J]. 化工进展, 2011,30(2):456-462. |
| [4] | 范剑明. 高铝煤矸石铝硅分级提取实验研究[J]. 无机盐工业, 2019,51(11):65-68. |
| [5] |
Guo Y, Yan K, Cui L, et al. Effect of Na2CO3 additive on the activa-tion of coal gangue for alumina extraction[J]. International Journal of Mineral Processing, 2014,131:51-57.
doi: 10.1016/j.minpro.2014.07.001 |
| [6] |
Han L, Ren W, Wang B, et al. Extraction of SiO2 and Al2O3 from coal gangue activated by supercritical water[J]. Fuel, 2019,253:1184-1192.
doi: 10.1016/j.fuel.2019.05.118 |
| [7] |
Yang Quancheng, Zheng Shili, Zhang Ran. Recovery of alumina from circulating fluidized bed combustion Al-rich fly ash using mild hydrochemical process[J]. Transactions of Nonferrous Metals Society of China, 2014,24:1187-1195.
doi: 10.1016/S1003-6326(14)63178-2 |
| [8] |
Ding J, Ma S, Xie Z, et al. Formation mechanism of an undesirable by-product in the mild hydro-chemical process for the extraction of alumina from fly ash and its mitigation[J]. Hydrometallurgy, 2019,186:292-300.
doi: 10.1016/j.hydromet.2019.04.012 |
| [9] | 李昌新, 喻源, 张庆武, 等. 合成条件对电解锰渣制备水化硅酸钙的溶解性能与溶解动力学的影响[J].应用化工, 2014(5):1-8. |
| [10] | 刘飞, 李静, 曹建新. 超轻微孔硅酸钙保温隔热材料的制备研究[J]. 新型建筑材料, 2010,37(7):21-24. |
| [11] | 伍泽广, 石松林, 刘钦甫. 煤系高岭土制备微孔硅酸钙[J].金属矿山, 2011(1):162-164. |
| [12] | 刘红艳, 王丽, 衣伟, 等. 利用工业电石渣合成硅酸钙保温材料[J]. 实验室研究与探索, 2010,29(1):9-11. |
| [13] |
Felipe Sesé M, Eliche Quesada D, Corpas Iglesias F A.The use of solid residues derived from different industrial activities to obtain calcium silicates for use as insulating construction materials[J]. Ceramics International, 2011,37(8):3019-3028.
doi: 10.1016/j.ceramint.2011.05.003 |
| [14] | 贺实月, 李会泉, 李少鹏, 等. 煤粉炉高铝粉煤灰碱溶脱硅反应动力学[J]. 中国有色金属学报, 2014,24(7):1888-1894. |
| [1] | Liu Xiaoting,Wen Jiuran,Wang Siyu,Liu Kaiping,Gao Ni,Zhong Jiaqiang. Study on strengthening technology of raw coal gangue aggregate [J]. Inorganic Chemicals Industry, 2020, 52(4): 65-71. |
| [2] | Wang Zinan,Chen Kui,Zhu Jiawen,Zhang Yurong,Lin Farong,Zhou Xiaokui. Effects of crystal seeds and salt dopants on phase transformation of TiO2 in calcination process [J]. Inorganic Chemicals Industry, 2020, 52(3): 45-50. |
| [3] | Xie Juan,Xia Runnan,Du Hongxia,Xu Yongquan,Zhao Shuchun,Xu Hong,Kang Wentong. Preparation of α-Fe2O3/coal gangue composite photocatalyst and its application in pentachlorophenol degradation [J]. Inorganic Chemicals Industry, 2019, 51(5): 74-77. |
| [4] | Fan Jianming1,2. Study on sequential extraction experiment of aluminum and silicon from high alumina coal gangue [J]. Inorganic Chemicals Industry, 2019, 51(11): 65-68. |
| [5] | ZHANG Jin-Shan, SUN Chun-Bao, CAO Zhao, FAN Wen-Yang, GUO Zhen-Kun, FAN Xue-Min. Experimental research on preparation of metakaolin from coal kaolinite [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(9): 64-. |
| [6] | RONG 尔Yi, ZHU Jia-Wen, CHEN Kui, YAO Heng-Ping, ZHOU Xiao-Kui. Effects of calcining seed,phosphate,and magnesium on titanium dioxide crystal [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(7): 21-. |
| [7] | FAN Wen-Yang, LI Xia, SUN Jian-Ling. Research on synthesis of 4A zeolite with a coal gangue in Inner Mongolia at low temperature [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(5): 44-. |
| [8] | ZHANG Xiang, DUAN Jian-Bang, LI Rui-Ge, CHEN Miao, LU Yan-Jie. Preparation of polyaluminum chloride from calcium reducing slag and coal gangue [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(4): 66-. |
| [9] | CHEN Wen-Xing, TIAN Juan, ZHOU Chang-Ping. Preparation technology of potassium sulfate with insoluble potassium contained shale [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(7): 42-. |
| [10] | . Experimental study on preparation of PAFS from acid leaching solution of high iron content coal gangue [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(6): 57-. |
| [11] | ZHAO Rui-Tong, WANG Jing, DUAN Xiao-Fang, FANG Li. Preparation technology of white carbon black from acid-leached residue of coal gangue [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(5): 53-. |
| [12] | WU Shi-Qin, LI Jun, JIN Yang, MA Yu-Jing, CHEN Xi. Effect of Fe3+, Mg2+, and Al3+ on phase transformation process of CaSO4·2H2O to CaSO4·0.5H2O in CaCl2-HCl-H2O electrolyte solution [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(4): 30-. |
| [13] | XUE Fang-Bin, YANG Xi, GUO Yan-Xia, YANG Feng-Ling, CHENG Fang-Qin. Preparation of AlCl3·6H2O from acid leached solution of coal gangue by HCl gas sparging [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(4): 47-. |
| [14] | KONG De-Shun, FAN Jia-Xin, SHI Kai-Yi, LI Zhi. Experimental study on preparation of iron oxide red from acid leaching solution of high-iron coal gangue [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(7): 56-. |
| [15] | ZHANG Lian-Ke, YANG Dong-Mei, Han-Jian-Hong, QU Tang-Chao. Study on removal of total phosphorus from eutrophic lake water by calcium silicate [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(11): 41-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|