Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (3): 45-50.doi: 10.11962/1006-4990.2019-0210
• Research & Development • Previous Articles Next Articles
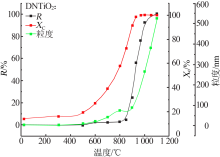
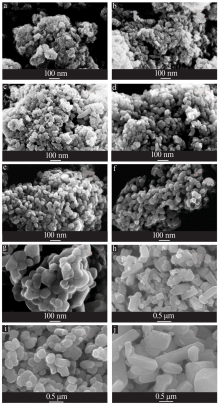
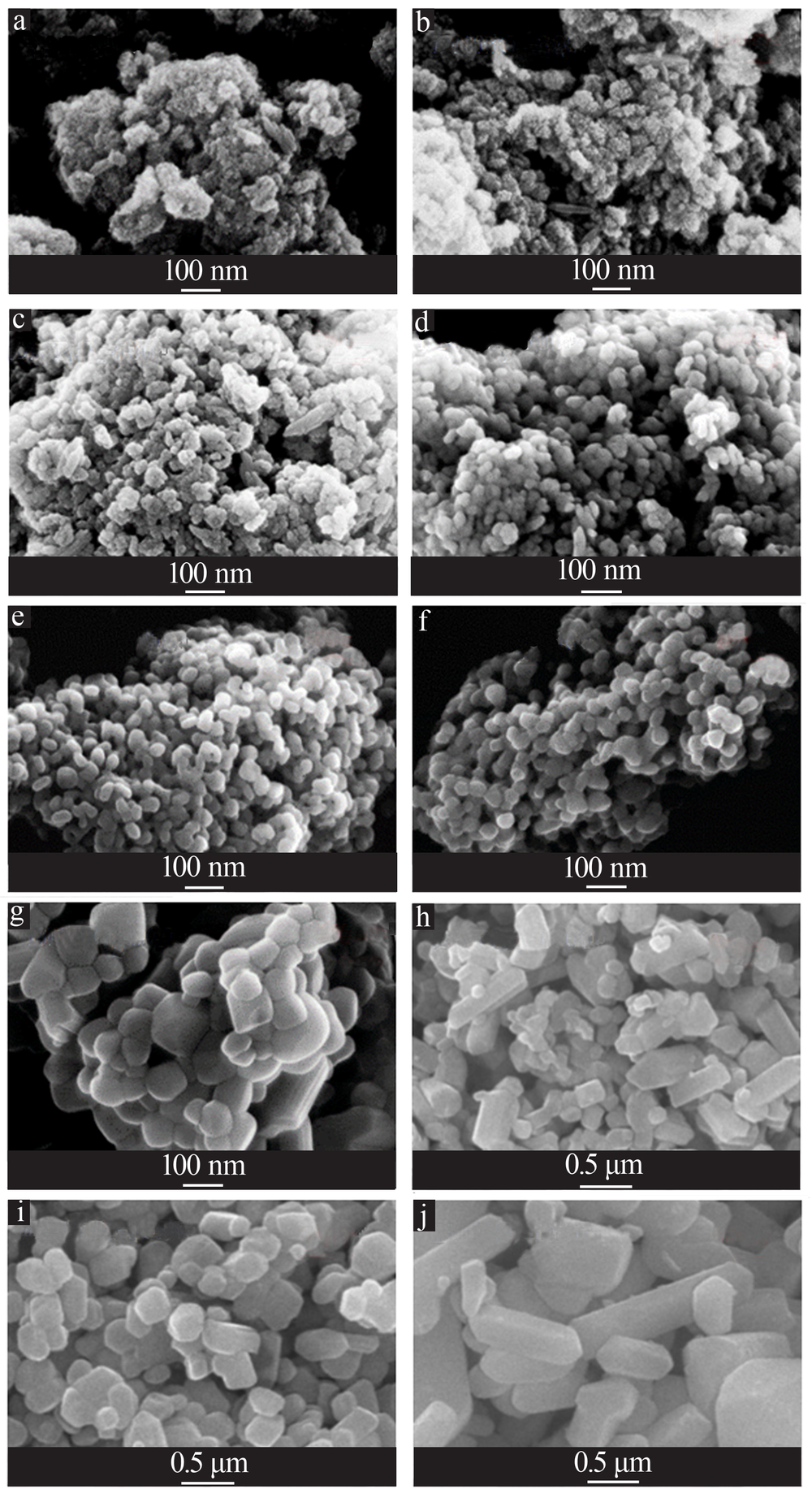
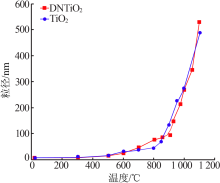
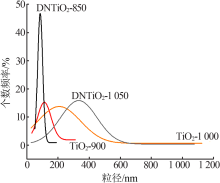
Effects of crystal seeds and salt dopants on phase transformation of TiO2 in calcination process
Wang Zinan1,Chen Kui1( ),Zhu Jiawen1,Zhang Yurong2,Lin Farong2,Zhou Xiaokui2
),Zhu Jiawen1,Zhang Yurong2,Lin Farong2,Zhou Xiaokui2
- 1. School of Chemical Engineering,East China University of Science and Technology,Shanghai 200237,China
2. Sichuan Lomon Corporation
-
Received:2019-09-26Online:2020-03-10Published:2020-03-31 -
Contact:Chen Kui E-mail:chenkui@ecust.edu.cn
CLC Number:
Cite this article
Wang Zinan,Chen Kui,Zhu Jiawen,Zhang Yurong,Lin Farong,Zhou Xiaokui. Effects of crystal seeds and salt dopants on phase transformation of TiO2 in calcination process[J]. Inorganic Chemicals Industry, 2020, 52(3): 45-50.
share this article
| [1] | 陈朝华, 刘长河 . 钛白粉生产及应用技术[M]. 北京: 化学工业出版社, 2005. |
| [2] | Hanaor D A H, Sorrell C C . Review of the anatase to rutile phase tr-ansformation[J]. Journal of Materials Science, 2011,46(4):855-874. |
| [3] | 黄超, 邓磊, 黄琼 , 等. 二氧化钛光催化剂降解气相苯系物的研究进展[J]. 无机盐工业, 2017,49(1):56-59. |
| [4] | 张宏忠, 张钰, 王明花 , 等. 二氧化钛光催化膜分离耦合技术在水处理中的应用[J]. 无机盐工业, 2017,49(7):50-54. |
| [5] | 唐波, 许妍霞, 宋兴福 , 等. 钛白酸解尾渣旋流分级数值模拟与实验研究[J]. 华东理工大学学报:自然科学版, 2016,42(4):454-459. |
| [6] | 朱容梅, 陈葵, 朱家文 , 等. 外加晶种对钛白粉水解过程及其亮度的影响[J]. 无机盐工业, 2019,51(2):30-34. |
| [7] | 刘翔, 朱家文, 陈葵 , 等. 钛液水解工艺对金红石型二氧化钛亮度的影响[J]. 无机盐工业, 2015,47(11):34-37. |
| [8] | 容尔益, 朱家文, 陈葵 , 等. 煅烧晶种和磷、镁对二氧化钛晶体的影响[J]. 无机盐工业, 2016,48(7):21-24. |
| [9] | Grzmil B, Kic B, Rabe M . Inhibition of the anatase-rutile phase trans-formation with addition of K2O,P2O5,and Li2O[J]. Chemical Papers, 2004,58(6):410-414. |
| [10] | Gesenhues U, Rentschler T . Crystal growth and defect structure of Al 3+-doped rutile [J]. Journal of Solid State Chemistry, 1999,143(2):210-218. |
| [11] | Takeuchi M, Martra G, Coluccia S , et al. Investigations of the struc-ture of H2O clusters adsorbed on TiO2 surfaces by near-infrared absorption spectroscopy[J]. The Journal of Physical Chemistry B, 2005,109(15):7387-7391. |
| [12] | Herman G S, Dohnálek Z, Ruzycki N , et al. Experimental investi-gation of the interaction of water and methanol with anatase-TiO2(101)[J]. The Journal of Physical Chemistry B, 2003,107(12):2788-2795. |
| [13] | Ginsberg T, Modigell M, Wilsmann W . Thermochemical characte-rization of the calcination process step in the sulphate method for production of titanium dioxide[J]. Chemical Engineering Research and Design, 2011,89(7):990-994. |
| [14] | 杨青 . Al盐对钛白粉窑下物形貌和颜料性能的影响[J]. 化工管理, 2017(33):85-86. |
| [1] | Guo Qiushuang,Wu Tongxu,Cai Zhe,Cai Qi,Li Xiaoyun,Sun Yanmin. Study on influence factors of specific surface area of Ti-Si composite oxides [J]. Inorganic Chemicals Industry, 2020, 52(12): 109-112. |
| [2] | Dong Xiongbo,Zhang Xiangwei,Liu Xiaorui,Zheng Shuilin. Progress and development trend of inorganic coated titanium powder [J]. Inorganic Chemicals Industry, 2020, 52(10): 30-36. |
| [3] | Gong Jiazhu. Future trend and development course of titanium dioxide pigment industry for sixty years in China [J]. Inorganic Chemicals Industry, 2020, 52(10): 55-63. |
| [4] | Xu Linchong,Yuan Aiqun,Wu Shengfu,Huang Zengwei,Bai Lijuan,Wei Dongping. Preparation process of core-shell structure TiO2@SiO2 optimized by response surface experiment [J]. Inorganic Chemicals Industry, 2020, 52(10): 77-83. |
| [5] | Gan Yonghai,Wu Bingdang,Li Haojie,Li Jingbiao,Li Runsheng,Zhang Shujuan. Preparation and application evaluation of a novel titanium coagulant [J]. Inorganic Chemicals Industry, 2020, 52(9): 1-5. |
| [6] | Zhang Shuli,Xu Xupeng,Chen Shurui,Liu Qianshu,Wang Benju. Photocatalytic activity of vanadium doped titanium dioxide [J]. Inorganic Chemicals Industry, 2020, 52(8): 93-97. |
| [7] | Peng Wenbo,Yun Jianjun,Ding Bangchao,Bai Zuguo,Xiao Weiyi,Wang Xiaohu. Study on membrane integration technology in treatment of wastewater from chlorinated titanium dioxide [J]. Inorganic Chemicals Industry, 2020, 52(6): 76-79. |
| [8] | Ji Lichun. Present status of resource utilization of by-product green vitriol from titanium dioxide production by sulfuric acid method [J]. Inorganic Chemicals Industry, 2020, 52(5): 11-17. |
| [9] | Gong Jiazhu,Lu Xiangfang,Wu Ninglan,Shao Guoxiong. Research and development of self-fitting nano-sized titanium dioxide catalytic wastewater treatment agent [J]. Inorganic Chemicals Industry, 2020, 52(5): 59-64. |
| [10] | Zhu Jiaxin,Xiong Yuhua,Guo Rui. Research progress in modification of TiO2 photocatalyst [J]. Inorganic Chemicals Industry, 2020, 52(3): 23-27. |
| [11] | Chen Shurui,Yang Shaoli,Ma Lan,Hou Jing. Study on leaching of titanium gypsum with hydrochloric acid [J]. Inorganic Chemicals Industry, 2020, 52(2): 65-68. |
| [12] | Chen Wen,Long Xiang,Li Haiyan,Yang Hui,Jia Guifa,Jin Yaqiu. Determination of particle size of gas phase oxided titanium dioxide by laser particle size analyzer [J]. Inorganic Chemicals Industry, 2020, 52(2): 69-71. |
| [13] | Gao Zengli,Li Qiuhong,Li Huaquan,Bi Hongyu. A new device for utilizing waste heat from calcination tail gas of titanium dioxide rotary kiln [J]. Inorganic Chemicals Industry, 2020, 52(2): 75-77. |
| [14] | Lu Ruifang,Wu Jianchun,Liu Chan. Influence of titanyl sulfate solution hydrolysis process on tintreducing power of rutile titanium dioxide [J]. Inorganic Chemicals Industry, 2019, 51(12): 44-48. |
| [15] | Zhang Hongguang1,Wang Guowei2,Sun Ge1,Xu Feng1,Li Hongmei1,Li Shuang1,Fu Shuang1. Study on relationship between luminescence and photocatalytic properties of titanium dioxide [J]. Inorganic Chemicals Industry, 2019, 51(12): 94-96. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|