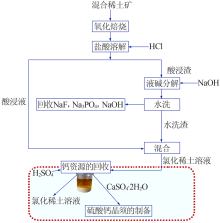
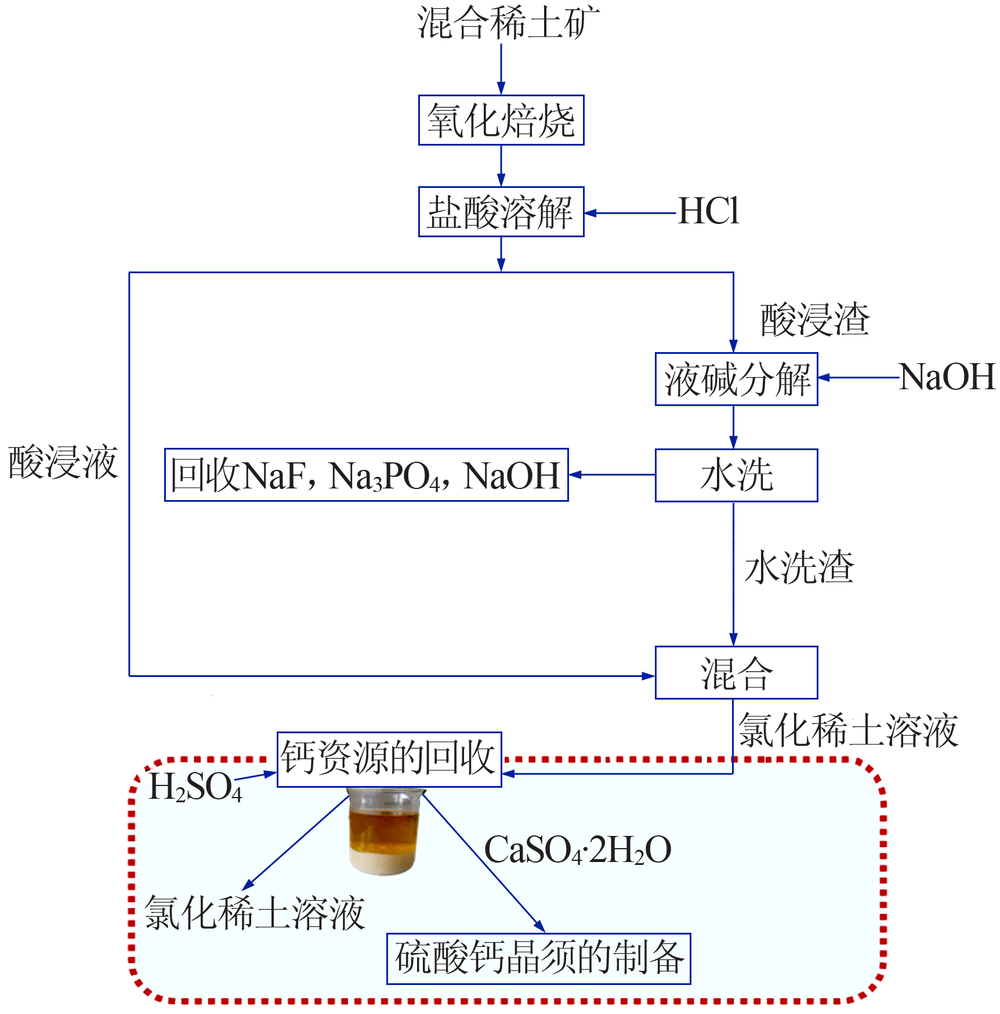
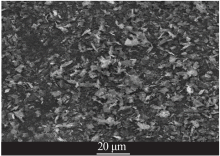
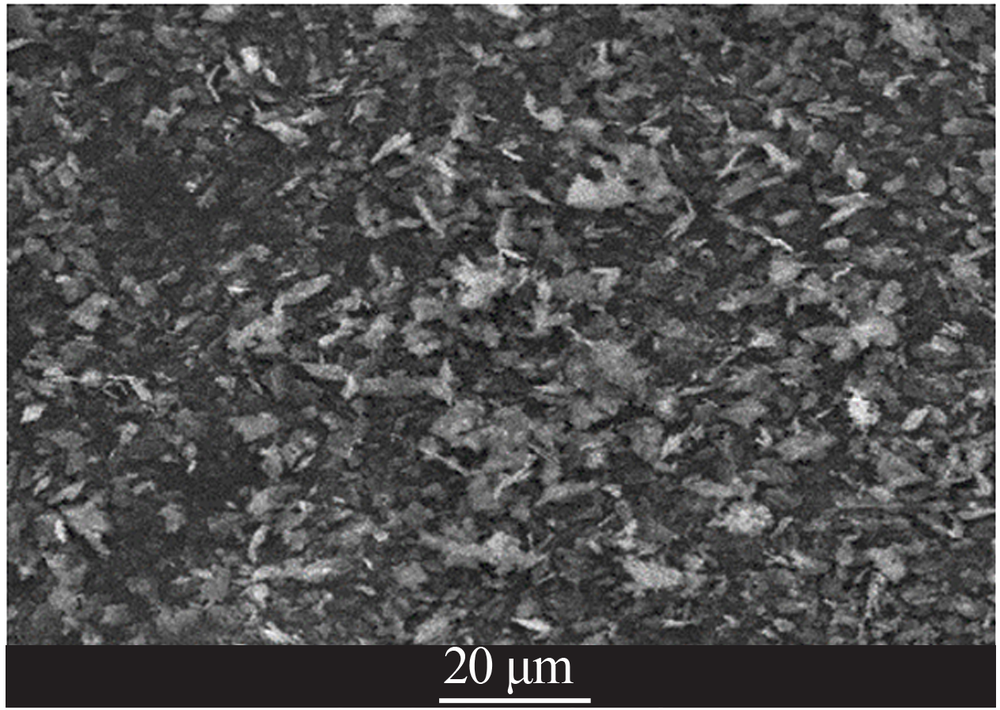
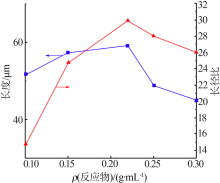
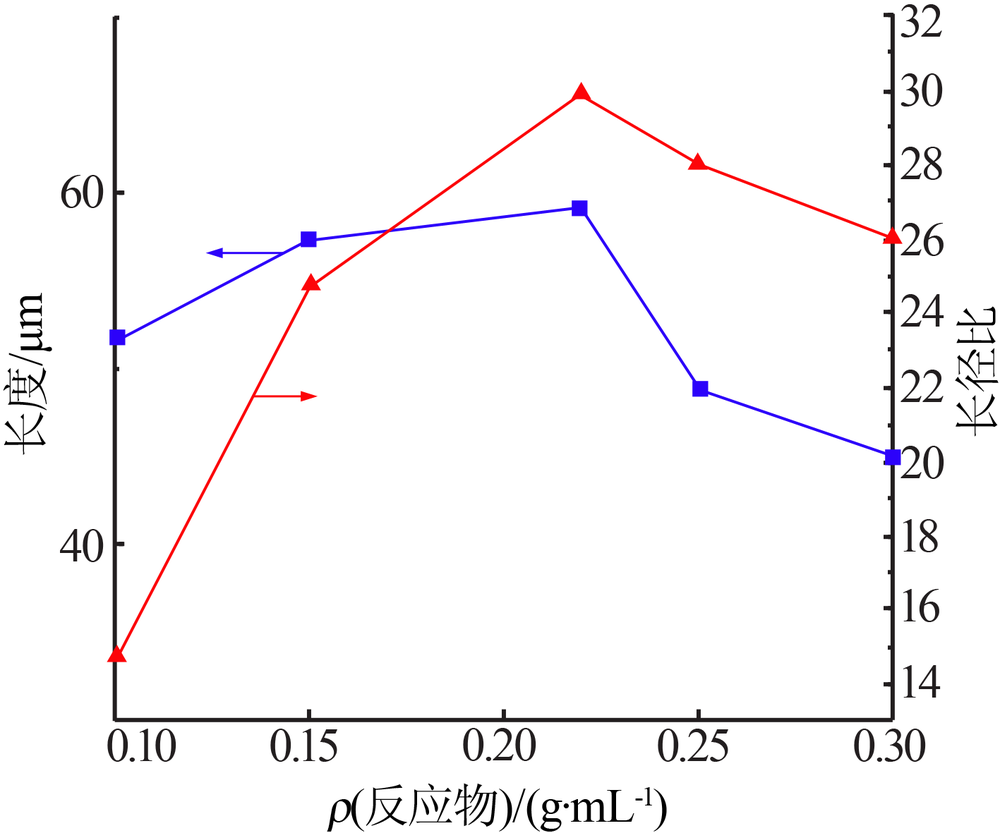
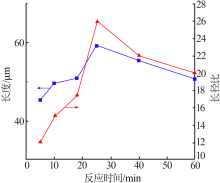
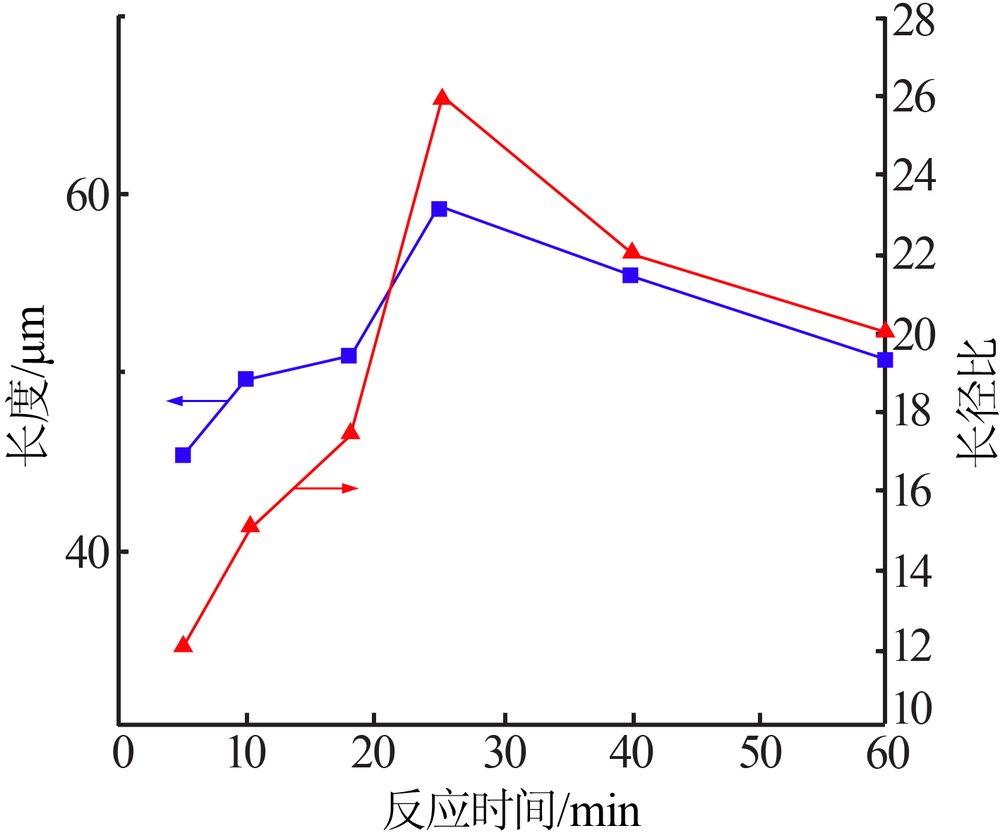
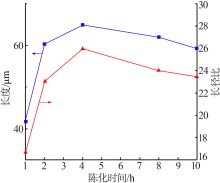
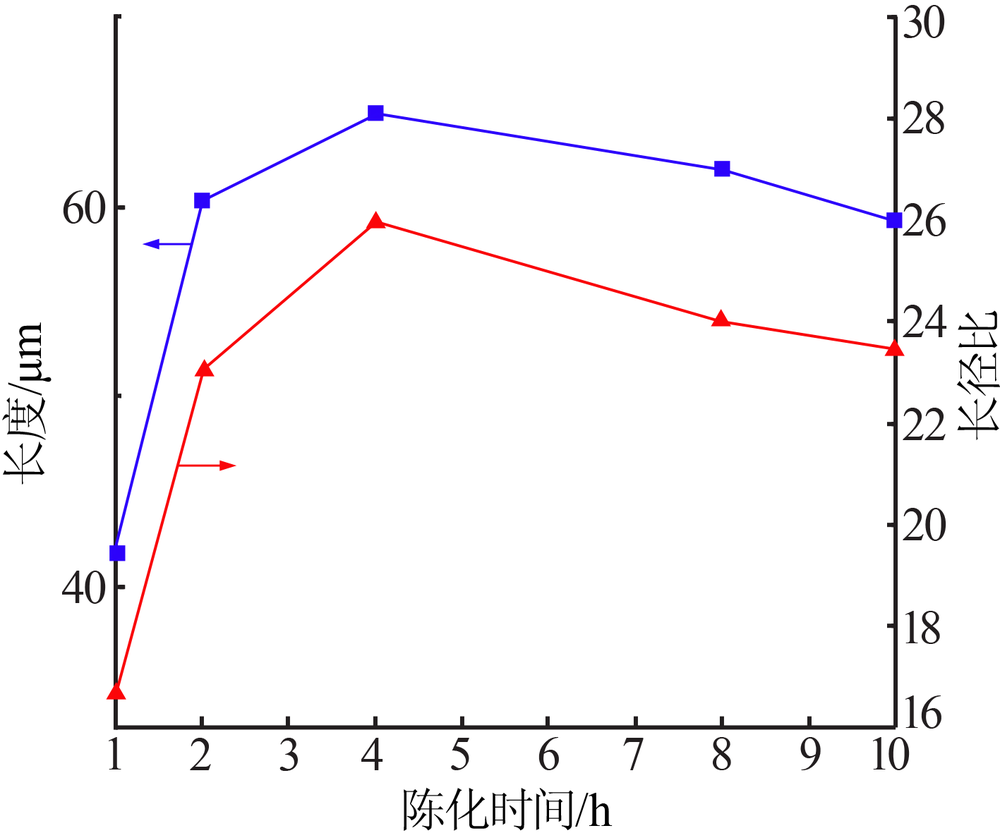
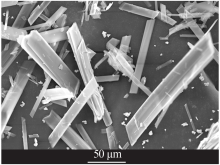
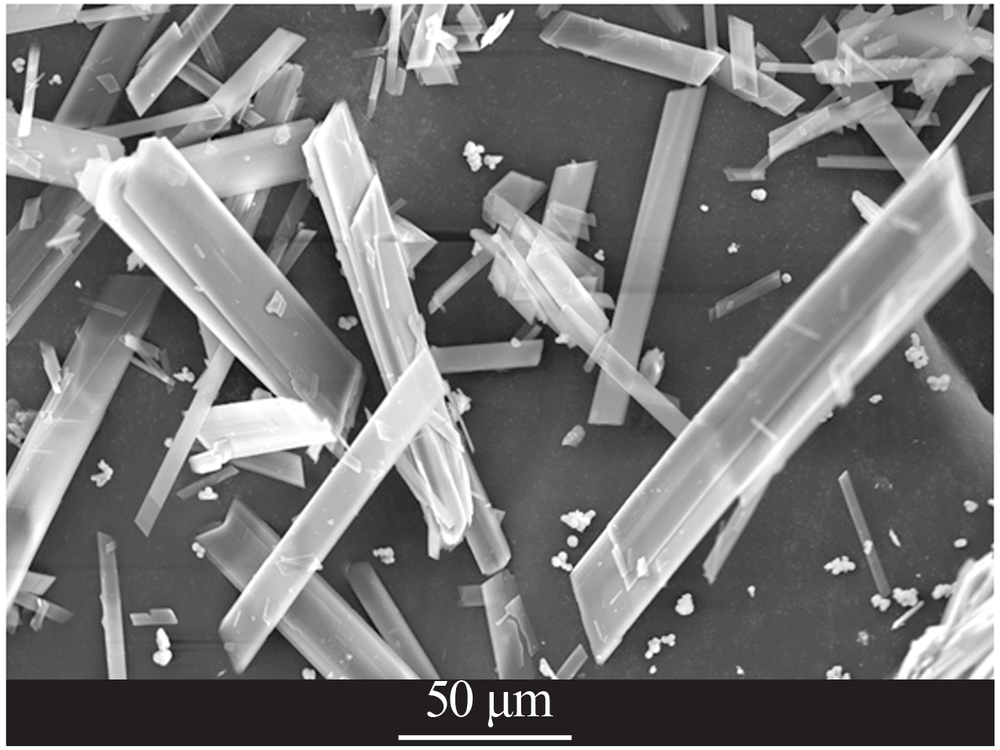
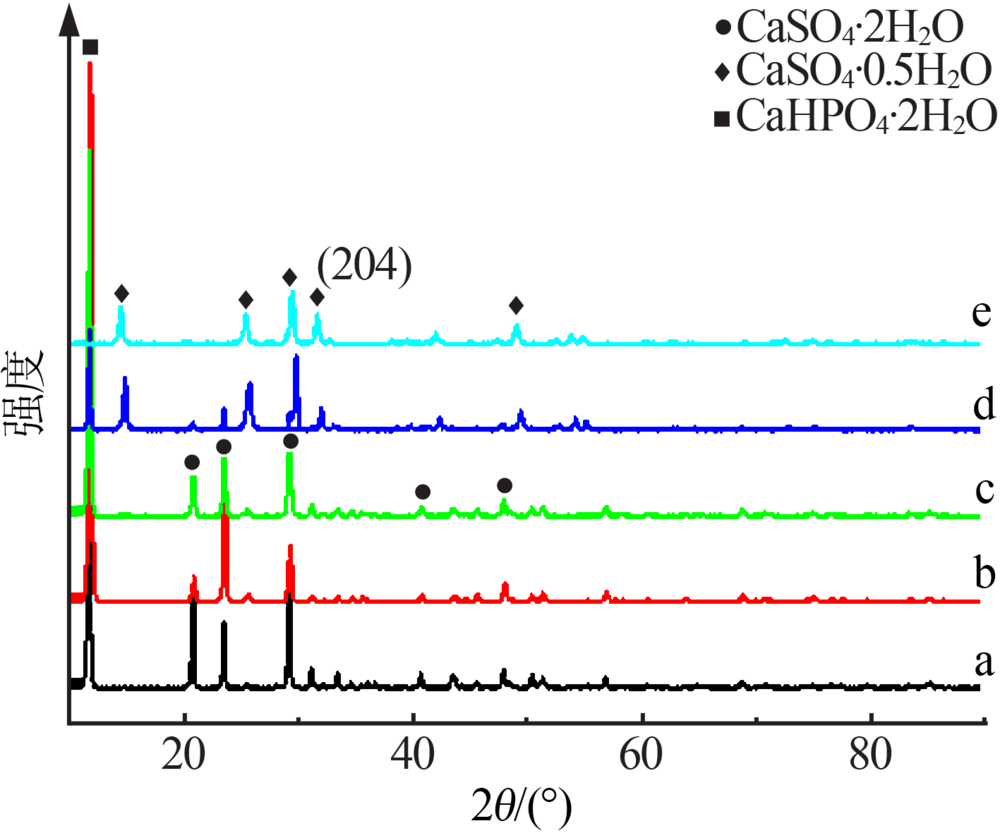
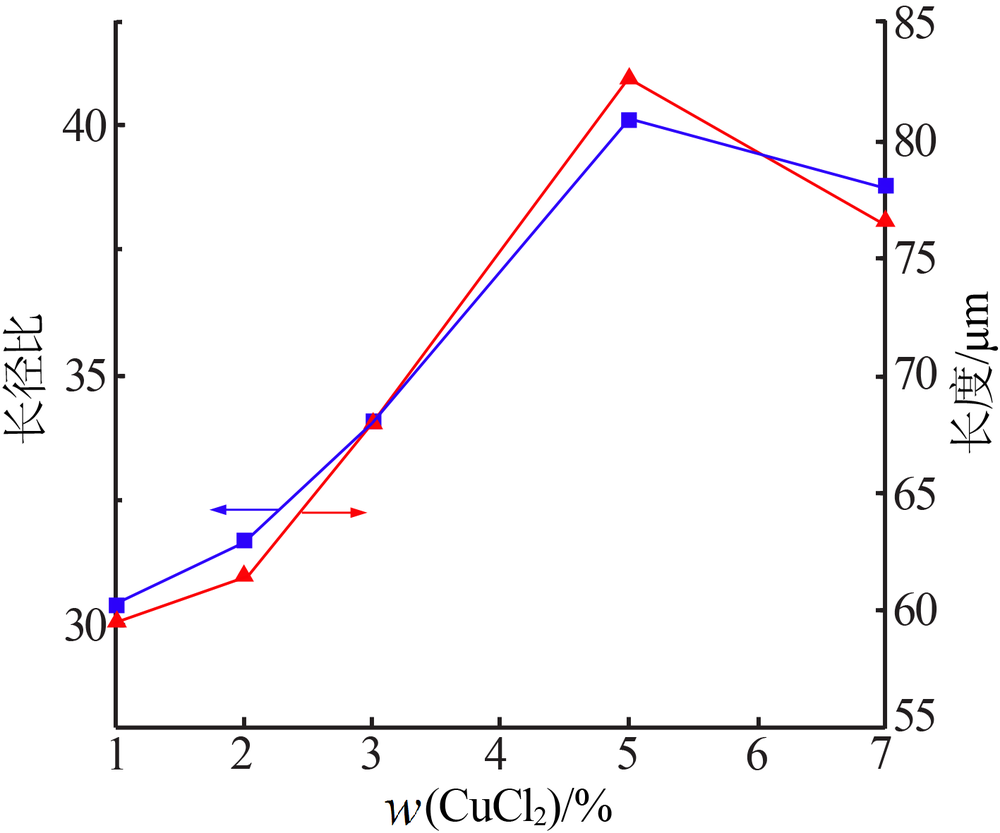
| [1] |
王舒州. 磷石膏利用液相生长法制备硫酸钙晶须的研究[D]. 绵阳:西南科技大学, 2017.
|
| [2] |
Sun H, Tan D, Peng T, et al. Preparation of calcium sulfate whisker by atmospheric acidification method from flue gas desulfurization gypsum[J]. Procedia Environmental Sciences, 2016,31:621-626.
|
| [3] |
李国勇, 崔益顺, 付凌杰. 磷石膏制备硫酸钙晶须的条件研究[J]. 无机盐工业, 2014,46(8):59-61.
|
| [4] |
吴叶, 高建明, 唐永波, 等. 脱硫石膏制备纳米级硫酸钙晶须的试验研究[J]. 新型建筑材料, 2018,45(1):96-100.
|
| [5] |
陈超, 张凡凡, 相利学, 等. 杂质对硝基磷石膏制备硫酸钙晶须形貌的影响[J]. 无机盐工业, 2017,49(7):61-64.
|
| [6] |
王浩, 成官文, 宋晓薇, 等. 化学沉淀法去除稀土湿法冶炼废水中钙与高浓度氨氮研究[J].环境科学, 2013(7):2718-2728.
|
| [7] |
贾江涛, 廖春生, 严纯华, 等. 萃取法Ca/La在线分离工艺[J]. 稀土, 1999,20(4):12-15.
|
| [8] |
黎新. 化学沉淀法去除稀土废水中钙镁的研究[D]. 西安:长安大学, 2016.
|
| [9] |
黎新, 孙长顺, 赵建军, 等. 分步沉淀法去除稀土废水中钙镁的研究[J].水处理技术, 2016(7):88-92.
|
| [10] |
Zhang D L, Li M, Gao K, et al. Physical and chemical mechanism underlying ultrasonically enhanced hydrochloric acid leaching of non-oxidative roasting of bastnaesite[J]. Ultrasonics Sonochemistry, 2017,39:774-781.
doi: 10.1016/j.ultsonch.2017.05.020
pmid: 28733006
|
| [11] |
宋景尧, 史培阳, 姜茂发, 等. 原料粒度对烧结烟气脱硫灰制备硫酸钙晶须的影响作用[J].粉煤灰综合利用, 2017(5):27-29.
|
| [12] |
刘凤铃, 彭佥赟, 刘洪, 等. 钛石膏制备CaSO4晶须[J].化工设计通讯, 2018(1):70-72.
|
| [13] |
甄德帅, 梁爽, 张兴文, 等. 硫酸钙晶须制备及应用综述[J]. 广东化工, 2017,44(11):140-141.
|
| [14] |
王露琦, 熊道陵, 李洋, 等. 硫酸钙晶须制备及应用研究进展[J]. 有色金属科学与工程, 2018,47(3):38-45.
|
| [15] |
侯炜, 王鸿康, 王少青, 等. 水热法制备硫酸钙晶须[J].内蒙古石油化工, 2017(3):16-18.
|
| [16] |
马园园. 氯化钙废液制备硫酸钙晶须工艺研究及工艺设计[D]. 石家庄:河北科技大学, 2018.
|
| [17] |
牟国栋. 半水石膏水化过程中的物相变化研究[J]. 硅酸盐学报, 2002,30(4):532-536.
|
| [18] |
杨荣华, 吴秀勇, 冯晓宁, 等. 用天然石膏制备硫酸钙晶须的研究[J]. 无机盐工业, 2010,42(1):44-47.
|
| [19] |
朱佳兵. 常压下石膏制备硫酸钙晶须工艺研究[D]. 成都:成都理工大学, 2016.
|
| [20] |
李前均, 谢燕, 陈前林, 等. 常压酸化法制备硫酸钙晶须工艺研究[J]. 无机盐工业, 2017,49(6):59-62.
|
| [21] |
吕智慧, 乃学瑛, 董亚萍, 等. 一步常压酸化法制备无水硫酸钙晶须[J]. 无机盐工业, 2017,49(1):22-24.
|
| [22] |
杨林, 周杰, 李贺军, 等. 晶形助长剂对磷石膏制硫酸钙晶须性能的影响[J]. 无机盐工业, 2012,44(10):41-43.
|
 ),Li Mei1,2(
),Li Mei1,2( ),Zhang Dongliang2,Gao Kai2,Li Jianfei2
),Zhang Dongliang2,Gao Kai2,Li Jianfei2