Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (7): 77-81.doi: 10.11962/1006-4990.2019-0440
• Chemical Analysis & Monitoring • Previous Articles Next Articles
Determination of BH4- concentration by iodometry and main influencing factors
Wu Bing
- Sanmenxia Quality Scientific Research Institute for Nonferrous Metals,Sanmenxia 472000,China
-
Received:2020-01-22Online:2020-07-10Published:2020-07-13
CLC Number:
Cite this article
Wu Bing. Determination of BH4- concentration by iodometry and main influencing factors[J]. Inorganic Chemicals Industry, 2020, 52(7): 77-81.
share this article
"
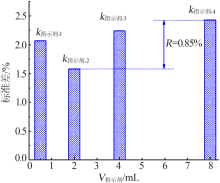
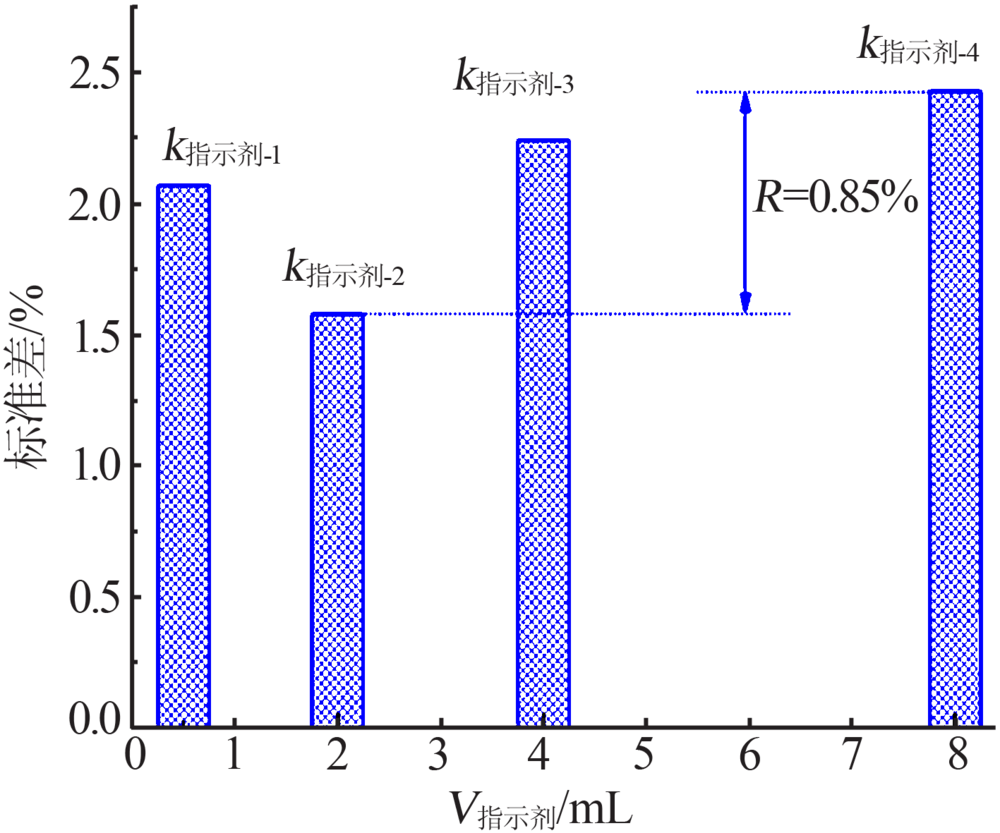
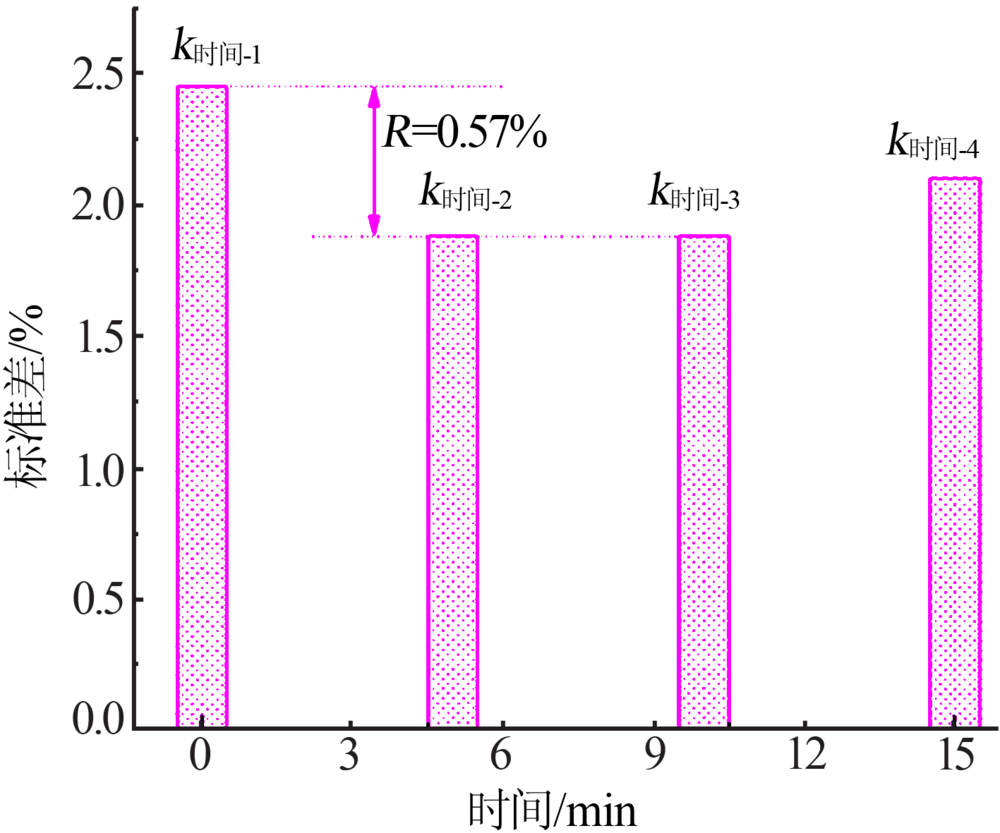
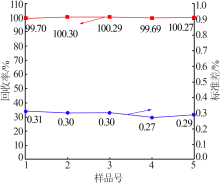
| 编号 | V/mL | nKI/mol | 时间/min | V指示剂/mL | 回收率/% | 标准差/% |
|---|---|---|---|---|---|---|
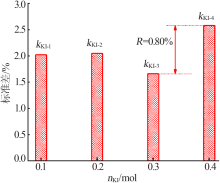
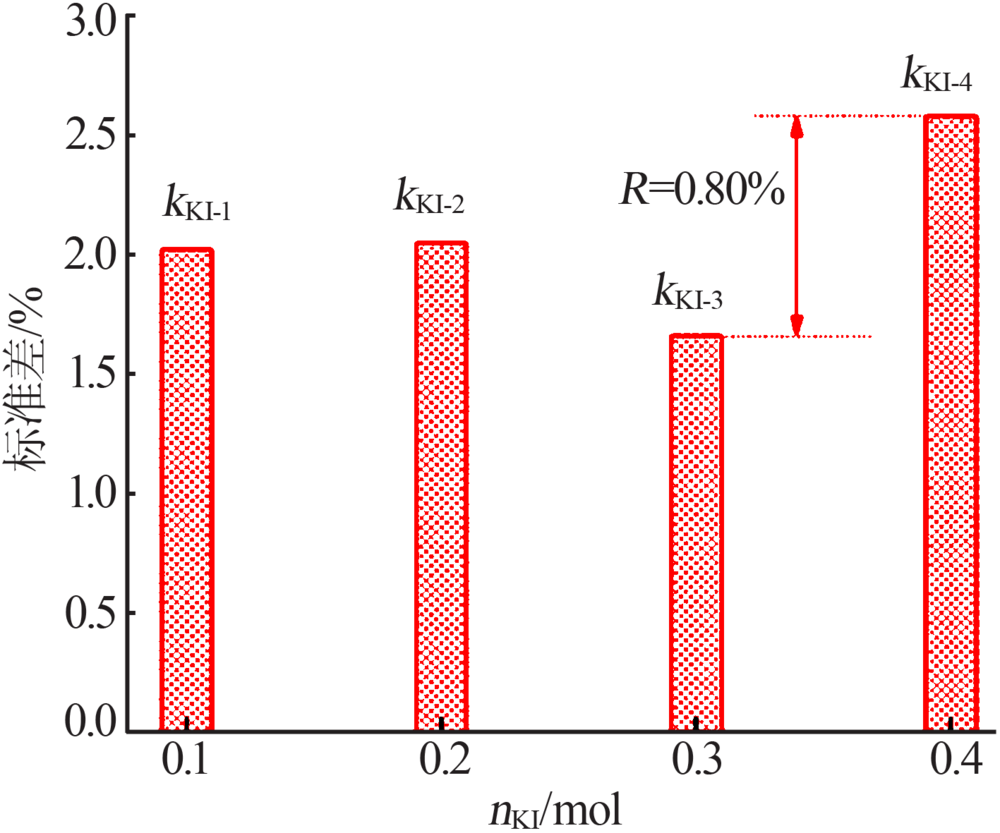
| 1 | 4.00 | 0.100 | 0 | 0.50 | 100.68 | 0.675 |
| 2 | 4.00 | 0.200 | 5 | 2.00 | 100.58 | 0.583 |
| 3 | 4.00 | 0.300 | 10 | 4.00 | 101.02 | 1.02 |
| 4 | 4.00 | 0.400 | 15 | 8.00 | 100.99 | 0.99 |
| 5 | 6.50 | 0.100 | 5 | 4.00 | 101.40 | 1.40 |
| 6 | 6.50 | 0.200 | 0 | 8.00 | 101.48 | 1.48 |
| 7 | 6.50 | 0.300 | 15 | 0.50 | 100.58 | 0.584 |
| 8 | 6.50 | 0.400 | 10 | 2.00 | 98.83 | 1.17 |
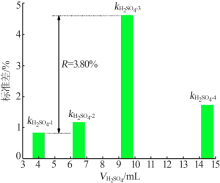
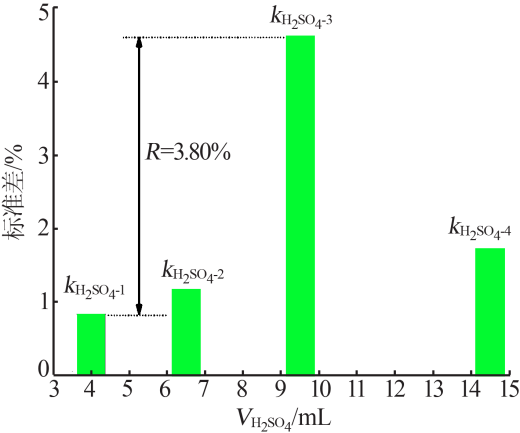
| 9 | 9.50 | 0.100 | 10 | 8.00 | 96.31 | 3.69 |
| 10 | 9.50 | 0.200 | 15 | 4.00 | 95.49 | 4.51 |
| 11 | 9.50 | 0.300 | 0 | 2.00 | 95.12 | 4.88 |
| 12 | 9.50 | 0.400 | 5 | 0.50 | 94.63 | 5.37 |
| 13 | 14.50 | 0.100 | 15 | 2.00 | 97.68 | 2.32 |
| 14 | 14.50 | 0.200 | 10 | 0.50 | 98.37 | 1.63 |
| 15 | 14.50 | 0.300 | 5 | 8.00 | 100.16 | 0.157 |
| 16 | 14.50 | 0.400 | 0 | 4.00 | 102.78 | 2.78 |
| [1] | 刘宇宏, 黄科林, 李克贤, 等. 硼氢化钠的性能与应用[J]. 企业科技与发展, 2009(24):20-23. |
| [2] | 白银娟, 路军, 马怀让. 硼氢化钠在有机合成中的研究进展[J]. 应用化学, 2002,19(5):409-415. |
| [3] | 车荣睿. 硼氢化钠及其在化学化工上的应用[J]. 广东化工, 1983(3):19-26. |
| [4] | 李红斌, 房桂干. 麦草APMP浆高白度漂白的研究Ⅱ—硼氢化钠的应用[J]. 中华纸业, 2008,29(24):44-47. |
| [5] | 闫雷, 于秀娟, 李淑琴, 等. 硼氢化钠还原法处理化学镀镍废液[J]. 化工环保, 2002,22(4):213-216. |
| [6] | Kojima Y, Suzuki K, Fukumoto K, et al. Hydrogen generation using sodium borohydride solution and metal catalyst coated on metal oxide[J]. Int.J.Hydrogen Energy, 2002,27(10):1029-1034. |
| [7] | Wang F C, Fang W H. The development of a PEMFC hybrid power electric vehicle with automatic sodium borohydride hydrogen generation[J]. Int.J.Hydrogen Energy, 2017,42(15):10376-10389. |
| [8] | Nunes H X, Ferreira M J F, Rangel C X, et al. Hydrogen generation and storage by aqueous sodium borohydride(NaBH4) hydrolysis for small portable fuel cells(H2-PEMFC)[J]. Int.J.Hydrogen Energy, 2016,41(34):15426-15432. |
| [9] |
Yi L, Wei W, Zhao C, et al. Electrochemical oxidation of sodium borohydride on carbon supported Pt-Zn nanoparticle bimetallic catalyst and its implications to direct borohydride-hydrogen peroxide fuel cell[J]. Electrochim.Acta, 2015,158:209-218.
doi: 10.1016/j.electacta.2015.01.111 |
| [10] | Hjelm R M E, Garsany Y, Atkinson R W, et al. Sodium borohydride oxidation on Pt and/or Pd-based electrodes in hydrogen peroxide direct borohydride fuel cells(H2O2-DBFCs)[J]. ECS Trans., 2017,80(8):1033-1042. |
| [11] | Mirkin M V, Bard A J. Voltammetric method for the determination of borohydride concentration in alkaline aqueous solutions[J]. Anal.Chem., 1991,63(5):532-533. |
| [12] |
Amendola S, Onnerud P, Kelly M T, et al. Inexpensive,in-situ monitoring of borohydride concentrations[J]. Talanta, 1999,49(2):267-270.
doi: 10.1016/s0039-9140(98)00387-7 pmid: 18967596 |
| [13] | 尤铁学. 碘量法标定二氧化硫和甲醛标准贮备溶液的改进[J]. 化学试剂, 2008,30(6):447-448. |
| [14] | 江志琴, 贵一夫, 罗郑琼, 等. 短碘量法连续测定铜精矿中铜、铁方法改进[J]. 铜业工程, 2019(1):121-124. |
| [15] | 庞文林. 氨水二次分离-碘量法测定铜锡钛合金中的铜含量[J]. 矿冶工程, 2018,38(5):119-121. |
| [16] | 胡永玫. 碘量法测定掺锑二氧化锡粉中锡[J]. 冶金分析, 2018,38(11):66-70. |
| [17] | 刘毅敏, 覃军, 王祥智, 等. 硼氢化钠样品纯度的测定[J]. 理化检验-化学分册, 2003,39(9):555-556. |
| [18] | Gyenge E L, Oloman C W. Electrosynjournal attempts of tetrahydridoborates[J]. J.Appl.Electrochem., 1998,28(10):1147-1151. |
| [19] | 邢书才, 杨永, 岳亚萍, 等. 碘量法滴定分析中影响分析质量因素的研究[J]. 中国测试, 2018,44(9):44-50. |
| [20] | Buleon A, Colonna P, Planchot V, et al. Starch granules:structure and biosynjournal[J]. Int.J.Biol.Macromol., 1998,23(2):85-112. |
| [1] | Hang Meiyan,Peng Yajuan,Liu Xinxin,Zhang Haiyan,Tao Xu. Quantitative study on influence of ferrochrome slag modification based on mortar strength decoupling method [J]. Inorganic Chemicals Industry, 2021, 53(1): 72-76. |
| [2] | Hou Lishuang,Gu Shouyu,Hou Cuihong,Wang Haobin,Li Luyi. Optimization of preparation process of sodium pyrophosphate chelates zinc by response surface analysis [J]. Inorganic Chemicals Industry, 2020, 52(7): 30-35. |
| [3] | Wang Jue,Shi Qifu,Zhou Junhong,Zhao Zengyi,Wang Fulong. Preliminary study on preparation of magnesium phosphite [J]. Inorganic Chemicals Industry, 2020, 52(6): 50-53. |
| [4] | Cheng Chunchun,Song Haoyu,Li Chunli. Analysis of material flow and energy flow of soda ash based on MFA [J]. Inorganic Chemicals Industry, 2020, 52(6): 59-62. |
| [5] | Li Jieen,He Binbin,Yang Xiushan,Xu Dehua,Zhang Zhiye. Study on phosphorus extraction and impurity reduction technology for pretreatment of middle-low grade phosphate ore [J]. Inorganic Chemicals Industry, 2020, 52(4): 33-36. |
| [6] | Liu Junjie,Peng Jun,Liu Lixia,An Shengli,Li Wenting. Progress in analytical methods of germaniumcontent [J]. Inorganic Chemicals Industry, 2020, 52(3): 17-22. |
| [7] | Chen Yuerong,Jin Huiming,Yu Liang,Shao Yangyang. Preparation of amorphous Co-Cr-B and catalytic sodium borohydride hydrolysis for hydrogen generation [J]. Inorganic Chemicals Industry, 2020, 52(2): 91-96. |
| [8] | Jin Jianhua,Li Yongyuan,Cai Xiangcuo. Optimization of preparation process of strontium aluminate long afterglow luminescent materials by response surface analysis [J]. Inorganic Chemicals Industry, 2020, 52(1): 59-62. |
| [9] | Zheng Shaocong,Wang Fan,Zhu Liping,Xu Chengkun. Experimental study on influence of phosphogypsum with modified phosphorus slag powder [J]. Inorganic Chemicals Industry, 2020, 52(1): 73-75. |
| [10] | Zhou Zhenjun,Zhang Hao,Luo Weihua,Xu Peijun,Cong Peiliang. Effect of hydrothermal ion exchange time on remodeling of leucite by cesium ion [J]. Inorganic Chemicals Industry, 2019, 51(9): 17-20. |
| [11] | Fang Yan,Dang Yagu,Zhang Shun. Economic analysis of sodium?鄄alkali desulfurization solution for improving double?鄄cycle alkali process [J]. Inorganic Chemicals Industry, 2019, 51(7): 58-60. |
| [12] | Zhang Pingjun,Yu Shujuan. Online analysis on effect of ethanol and ultrasonic wave on crystal nucleus of potassium sulfate [J]. Inorganic Chemicals Industry, 2019, 51(6): 65-68. |
| [13] | Sun Haijie,Chen Lingxia,Zhang Yufeng,An Dongdong,Liu Cong. Performance of Co-B/ZrO2 catalyst for hydrogen generation from catalytic hydrolysis of sodium borohydride solution [J]. Inorganic Chemicals Industry, 2019, 51(3): 72-76. |
| [14] | Liu Wenhui. Research on characteristics and modification performance of environmentalfriendly inorganic precipitating diversion agent [J]. Inorganic Chemicals Industry, 2019, 51(11): 23-27. |
| [15] | Li Jie1,Cao Ying1,Yang Dongzhao2,Meng Hao1,Li Ru1,Xu Mingze1. Analysis and precautions safety risks for chlorine turbine compressor [J]. Inorganic Chemicals Industry, 2019, 51(10): 81-83. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|