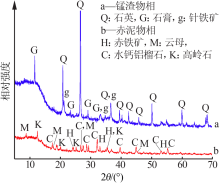
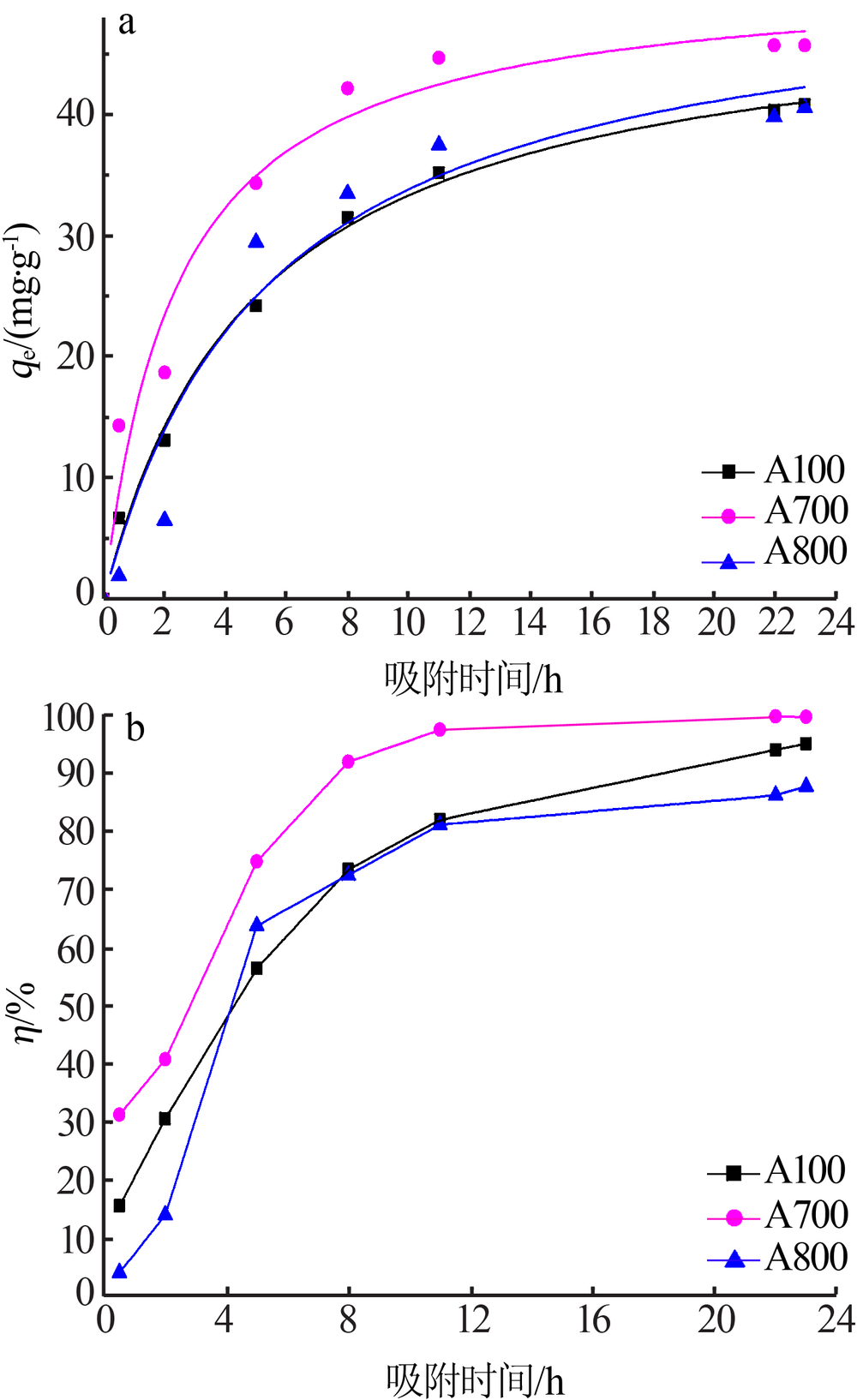
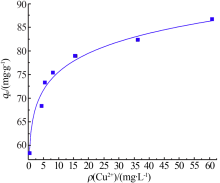
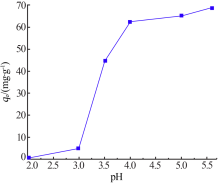
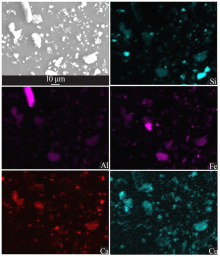
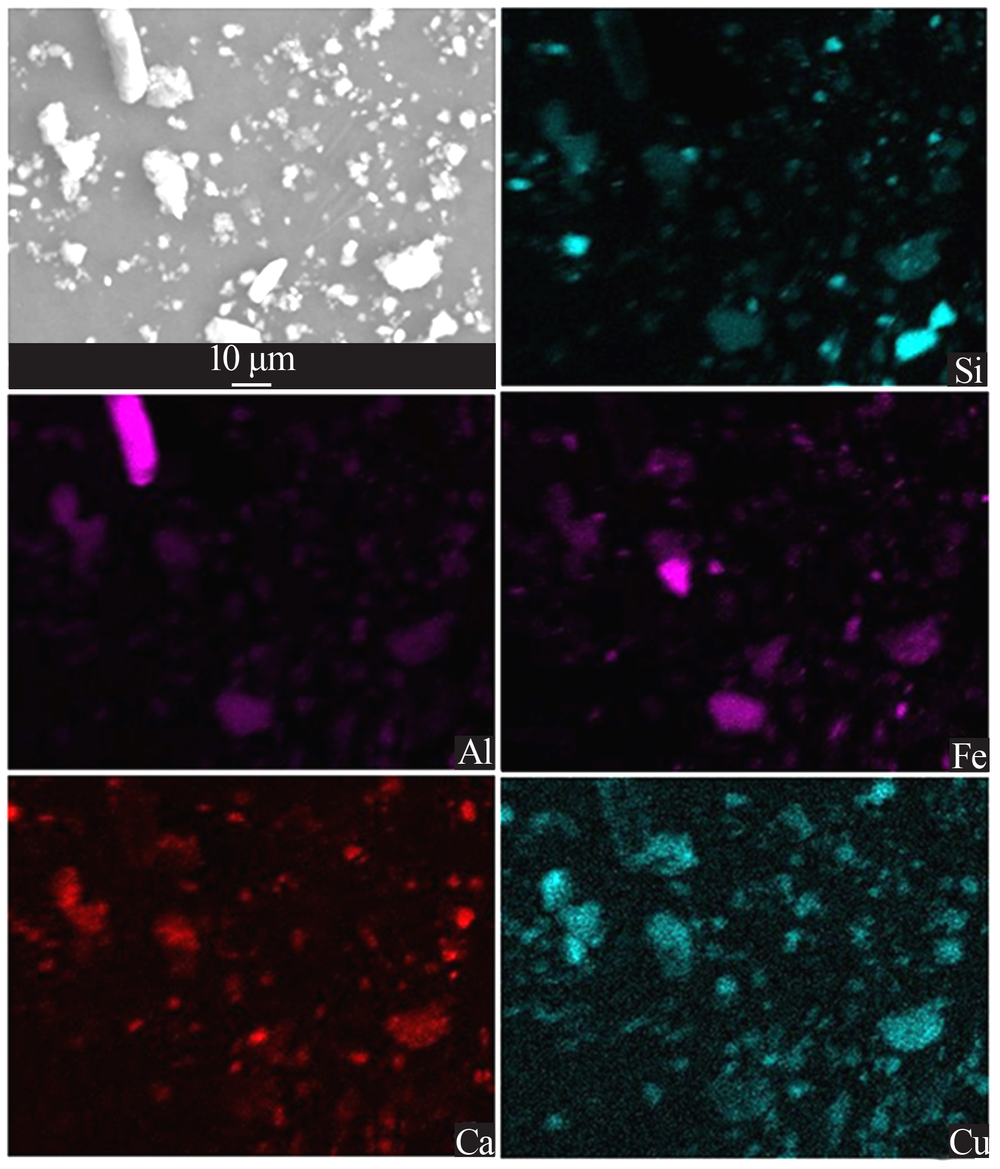
| [1] |
吴建锋, 宋谋胜, 徐晓虹 , 等. 电解锰渣的综合利用进展与研究展望[J]. 环境工程学报, 2014,8(7):2645-2652.
|
| [2] |
彭秋菊, 李佳, 叶恒朋 , 等. 电解锰渣中低活性硅的活化工艺研究[J]. 非金属矿, 2017,40(6):23-26.
|
| [3] |
Yang Y Q, Gu H N, Guo T F , et al. Environmental assessment of manganese sulfate residues derived from pyrolusite process[J]. Fresenius Environmental Bulletin, 2018,27(7):4883-4888.
|
| [4] |
周长波, 何捷, 孟俊利 , 等. 电解锰废渣综合利用研究进展[J]. 环境科学研究, 2010,23(8):1044-1048.
|
| [5] |
杨永琼, 张耀, 李晓燕 . 赤泥中重金属元素的浸出性与结合形态[J]. 化工环保, 2018,38(2):227-230.
|
| [6] |
Gu H N, Wang N, Liu S . Radiological restrictions of using red mud as building material additive[J]. Waste Management & Research, 2012,30(9):961-965.
|
| [7] |
Deng B, Li G, Luo J , et al. Enrichment of Sc2O3 and TiO2 from baux-ite ore residues[J]. Journal of Hazardous Materials, 2017,331:71-80.
|
| [8] |
Liu W, Chen X, Li W , et al. Environmental assessment,management and utilization of red mud in China[J]. Journal of Cleaner Produc-tion, 2014,84:606-610.
|
| [9] |
赵玉莲, 刘敬, 何瑞明 , 等. 赤泥还原焙烧磁选回收铁的试验研究[J]. 材料研究与应用, 2017,11(4):256-263,268.
|
| [10] |
费欣宇, 李海燕, 罗和亿 , 等. 赤泥基陶粒的制备及性能研究[J]. 非金属矿, 2017,40(5):9-12.
|
| [11] |
Guo T F, Yang H Q, Liu Q Y , et al. Adsorptive removal of phosp-hate from aqueous solutions using different types of red mud[J]. Water Science and Technology, 2018,2017(2):570-577.
|
| [12] |
朱丽, 李晔, 张猛 , 等. 硝酸铈改性赤泥制备除磷吸附剂[J]. 化工环保, 2012,32(1):81-84.
|
| [13] |
史力争, 陈惠康, 吴川 , 等. 赤泥及其复合钝化剂对土壤铅、镉和砷的稳定效应[J]. 中国科学院大学学报, 2018,35(5):617-626.
|
| [14] |
邵红, 张扬, 孙伶 . 铁硅改性膨润土对废水有机污染物的吸附性能研究[J]. 辽宁化工, 2005,34(9):391-394.
|
| [15] |
王芳, 罗琳, 易建龙 , 等. 赤泥质陶粒吸附模拟酸性废水中铜离子的行为[J]. 环境工程学报, 2016,10(5):2440-2446.
|
| [16] |
陆爱华 . 赤泥对含铜废水的吸附性能研究[J]. 山东化工, 2017,46(14):178-181.
|
| [17] |
张启亮, 张咏晶, 徐艳 , 等. Cu 2+和Cd 2+对活性污泥吸附Pb 2+的竞争吸附影响效果研究 [J]. 安徽农业科学, 2012,40(14):8258-8262.
|
| [18] |
卢仪思, 王明明, 黄耕 , 等. 改性赤泥处理酸性矿井废水的试验研究[J]. 非金属矿, 2018,41(6):15-18.
|
| [19] |
Yang F, Zhang S, Li H , et al. Corn straw-derived biochar impregna-ted with alpha-FeOOH nanorods for highly effective copper remo-val[J]. Chemical Engineering Journal, 2018,348:191-201.
|
| [20] |
Peng S, Wang R, Yang L , et al. Biosorption of copper,zinc,cadmi-um and chromium ions from aqueous solution by natural foxtail millet shell[J]. Ecotoxicology and Environmental Safety, 2018,165:61-69.
|
 ),Guo Tengfei2,3,Dai Yang2,3,Wang Ning2
),Guo Tengfei2,3,Dai Yang2,3,Wang Ning2