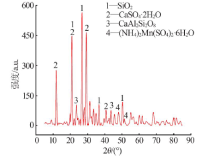
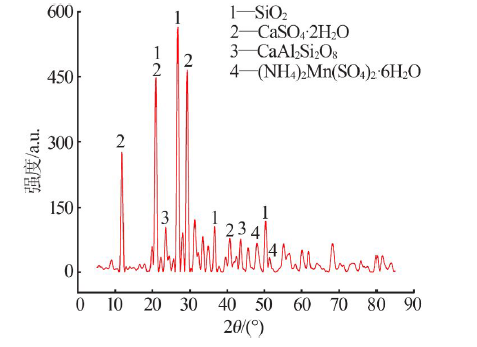
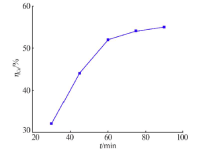
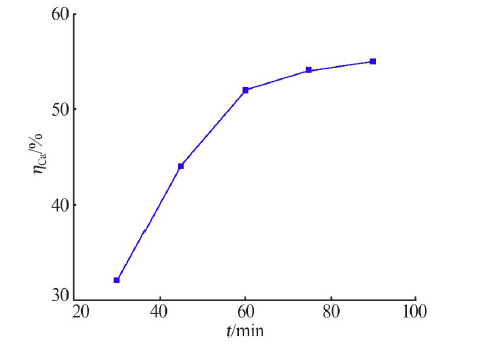
Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (1): 82-86.doi: 10.11962/1006-4990.2020-0074
Previous Articles Next Articles
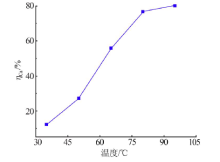
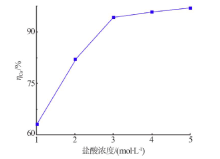
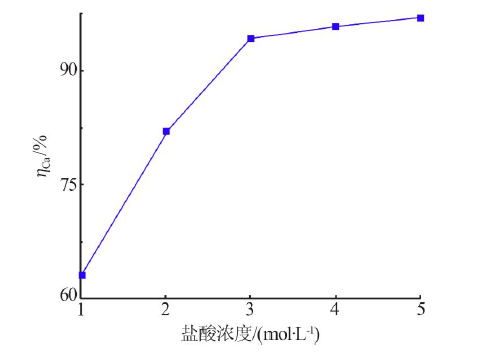
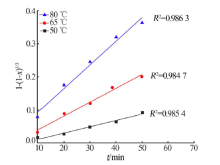
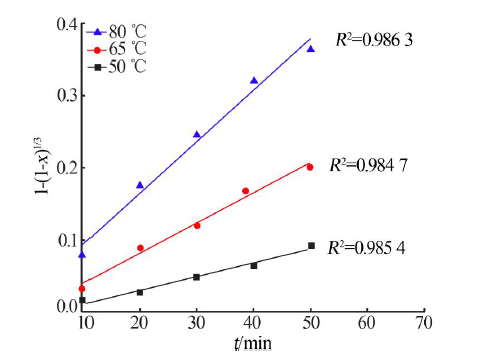
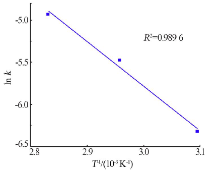
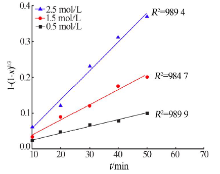
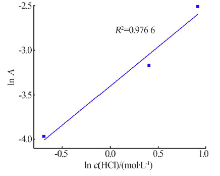
Kinetics study of calcium leaching from electrolytic manganese residue by hydrochloric acid
Yang Xiaohong1( ),Xue Xishi2,Zhang Lulu1,Chang Jun1
),Xue Xishi2,Zhang Lulu1,Chang Jun1
- 1. School of Materials and Chemical Engineering,Tongren University,Tongren 554300,China
2. Guizhou Kaisheng Potassium Co.,Ltd.
-
Received:2020-07-24Online:2021-01-10Published:2021-01-08 -
Contact:Yang Xiaohong E-mail:yangxiaohong201101@163.com
CLC Number:
Cite this article
Yang Xiaohong,Xue Xishi,Zhang Lulu,Chang Jun. Kinetics study of calcium leaching from electrolytic manganese residue by hydrochloric acid[J]. Inorganic Chemicals Industry, 2021, 53(1): 82-86.
share this article
| [1] | 张超, 王帅, 钟宏, 等. 电解锰渣无害化处理与资源化利用技术研究进展[J]. 矿产保护与利用, 2019(3):111-118. |
| [2] | Shu J, Liu R, Liu Z, et al. Solidification/stabilization of electrolytic manganese residue using phosphate resource and low-grade MgO/CaO[J]. J.Hazard.Mater., 2016,317:267-274. |
| [3] | Wang Y, Gao S, Liu X, et al. Preparation of nonsintered permeable bricks using electrolytic manganese residue:environmental and NH3-N recovery benefits[J]. J.Hazard.Mater., 2019,378:120768. |
| [4] | 牛莎莎, 王志兴, 郭华军, 等. 电解锰阳极渣还原浸出锰[J]. 中国有色金属学报, 2012,22(9):2662-2666. |
| [5] | 高武斌, 王志增, 赵伟洁, 等. 电解锰渣复合Fe-Mn-Cu-Co系红外辐射材料的制备及性能研究[J]. 功能材料, 2015,46(6):6076-6080. |
| [6] | 王积伟, 周长波, 杜兵, 等. 电解锰渣无害化处理技术[J]. 环境工程学报, 2014,8(1):329-333. |
| [7] | Shu J, Chen M, Wu H, et al. An innovative method for synergistic stabilization/solidification of Mn2+,NH4+-N,PO43-and F- in electro-lytic manganese residue and phosphogypsum[J]. J.Hazard.Mater., 2019,376:212-222. |
| [8] |
Wang N, Fang Z, Peng S, et al. Recovery of soluble manganese from electrolyte manganese residue using a combination of ammonia and CO2[J]. Hydrometallurgy, 2016,164:288-294.
doi: 10.1016/j.hydromet.2016.06.019 |
| [9] | 金修齐, 黄代宽, 赵书晗, 等. 电解锰渣胶凝固化研究进展及其胶结充填可行性探讨[J]. 矿物岩石地球化学通报, 2019,38:1-8. |
| [10] | Duan N, Wang F, Zhou C B, et al. Analysis of pollution materials generated from electrolytic manganese industries in China[J]. Re-sources,Conservation and Recycling, 2010,54(8):506-511. |
| [11] |
Hu N, Zheng J F, Ding D X, et al. Metal pollution in Huayuan river in Hunan province in China by manganese sulphate waste resi-due[J]. Bulletin of Environmental Contamination and Toxicology, 2009,83(4):583-590.
doi: 10.1007/s00128-009-9802-9 |
| [12] |
Zhou C B, Wang N F. Treating electrolytic manganese residue with alkaline additives for stabilizing manganese and removing ammo-nia[J]. Korean Journal of Chemical Engineering, 2013,30(11):2037-2042.
doi: 10.1007/s11814-013-0159-8 |
| [13] | 王智, 孙军, 钱觉时, 等. 电解锰渣中硫酸盐性质的研究[J]. 材料导报, 2010,24(10):61-64. |
| [14] | 张超, 王帅, 钟宏. 电解锰渣无害化处理与资源化利用技术研究进展[J]. 矿产保护与利用, 2019(3):111-118. |
| [15] | 吴建锋, 宋谋胜, 徐晓虹, 等. 电解锰渣的综合利用进展与研究展望[J]. 环境工程学报, 2014,8(7):2645-2652. |
| [16] | 朱炳晨. 化学反应工程[M]. 北京: 化学工业出版社, 2011: 212. |
| [17] |
Zhu Xiaobo, Wang Li, Guan Xuemao. Kinetics of titanium leaching with citric acid in sulfuric acid from red mud[J]. Transactions of Nonferrous Metals Society of China, 2015,25(9):3139-3145.
doi: 10.1016/S1003-6326(15)63944-9 |
| [18] |
Kavci E, Calban T, Colak S, et al. Leaching kinetics of ulexite in sodium hydrogen sulphate solutions[J]. Journal of Industrial and Engineering Chemistry, 2014,20(5):2625-2631.
doi: 10.1016/j.jiec.2013.12.089 |
| [19] | 黄博云, 颜文斌, 周再兴. 铁酸锌的酸浸动力学及含铁酸锌废料浸出研究[J]. 冶金工程, 2017,4(1):61-69. |
| [20] | Shu C Y, Alan B, David R S. Kinetic characterization for dilute sul-furic acid hydrolysis of timber varieties and switchgrass[J]. Biore-source Technology, 2008,99(9):3855-3863. |
| [21] | 陈毛毛, 陈莉荣. 铝矿厂赤泥硫酸浸出动力学研究[J]. 材料导报, 2015,29(18):141-145. |
| [1] | Liu Yuanhui,Tang Xiaona,Xie Lei,Jiang Xiaopeng,Zhang Yunbo. Investigation progress of ageing process of plaster of paris [J]. Inorganic Chemicals Industry, 2020, 52(9): 15-20. |
| [2] | Wang Haohao,Da Tao,Ye Shichao. Experimental study on crystallization process of ammonium chloride [J]. Inorganic Chemicals Industry, 2020, 52(4): 49-52. |
| [3] | Chen Shurui,Yang Shaoli,Ma Lan,Hou Jing. Study on leaching of titanium gypsum with hydrochloric acid [J]. Inorganic Chemicals Industry, 2020, 52(2): 65-68. |
| [4] | Zhu Huixia,Zhang Ting,Han Cong,Sun Lina,Li Ling. Study on adsorption performance of K-feldspar loaded MnO2 for Ni 2+ [J]. Inorganic Chemicals Industry, 2020, 52(1): 44-48. |
| [5] | Liu Sihao,Chen Jinfang,Wang Yan. Study on technological condition and kinetic of preparing manganese(Ⅲ) pyrophosphate by electrolysis [J]. Inorganic Chemicals Industry, 2019, 51(9): 24-29. |
| [6] | Chang Jun,Jia Fukang,Hu Chengshan,Ye Qianxu. Adsorption of manganese ion by zeolite synthesized from electrolytic manganese residue [J]. Inorganic Chemicals Industry, 2019, 51(9): 61-66. |
| [7] | Li Ying,Chen Xia,Miao Shulan,Liao Enxin. Study on adsorption properties of ammonium phosphate tungsten composites on rubidium and cesium [J]. Inorganic Chemicals Industry, 2019, 51(6): 34-37. |
| [8] | Zhou Hongyan,Chen Ping,Zhao Yanrong,Liu Rongjin,Wei Jiazhan. Sulfate activation of electrolytic manganese residue on heat-stewed steel slag activity [J]. Inorganic Chemicals Industry, 2019, 51(5): 66-69. |
| [9] | ZHOU Fang, CHEN Yu-Ting, XU Yuan-Lai, CHI Ru-An. Preparation and kinetic research of aluminum hypophosphite [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(8): 21-. |
| [10] | JIN Jian-Hua, YANG Zhan-Shou, LI Ao, MA Jiang-Feng. Experimental research on key parameters of preparation of strontium salt by hydrochloric acid leaching technology with waste strontium slag [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(8): 60-. |
| [11] | JIANG Chun-Li, SONG Hong-Jun, HAN Biao, ZHAO Wei, DONG Li-Chun, HUANG Shi-You, WEN Feng-Yu. Production process of extraction chromium from chromite with sub-molten sodium hydroxide [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(7): 39-. |
| [12] | ZUO Wei-Yuan, TONG Hai-Juan, SHI Bing-Fang. Adsorption of Mn(Ⅱ) by bentonite-activate carbon compound adsorbent [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(7): 58-. |
| [13] | XIE Wei, HUANG Run-Jun, CHEN Dong-Lian, HUANG Zeng-Wei, MA Shao-Mei, YUAN Ai-Qun. Adsorption kinetics of aluminum tripolyphosphate to ammonia nitrogen in water [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(6): 54-. |
| [14] | CAO Jing, QIAO Xiu-Chen, LIU Cheng-Liang, ZHANG Jing-Shi. A study on kinetics of limestone decomposition in air and CO2 atmospheres [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(12): 32-. |
| [15] | SHI Qin, HUANG Xue-Li. Thermodynamic simulation and kinetics experiment of K+ adsorption by merlinoite [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(11): 12-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|