Inorganic Chemicals Industry ›› 2019, Vol. 51 ›› Issue (6): 49-52.
• Environment·Health·Safety • Previous Articles Next Articles
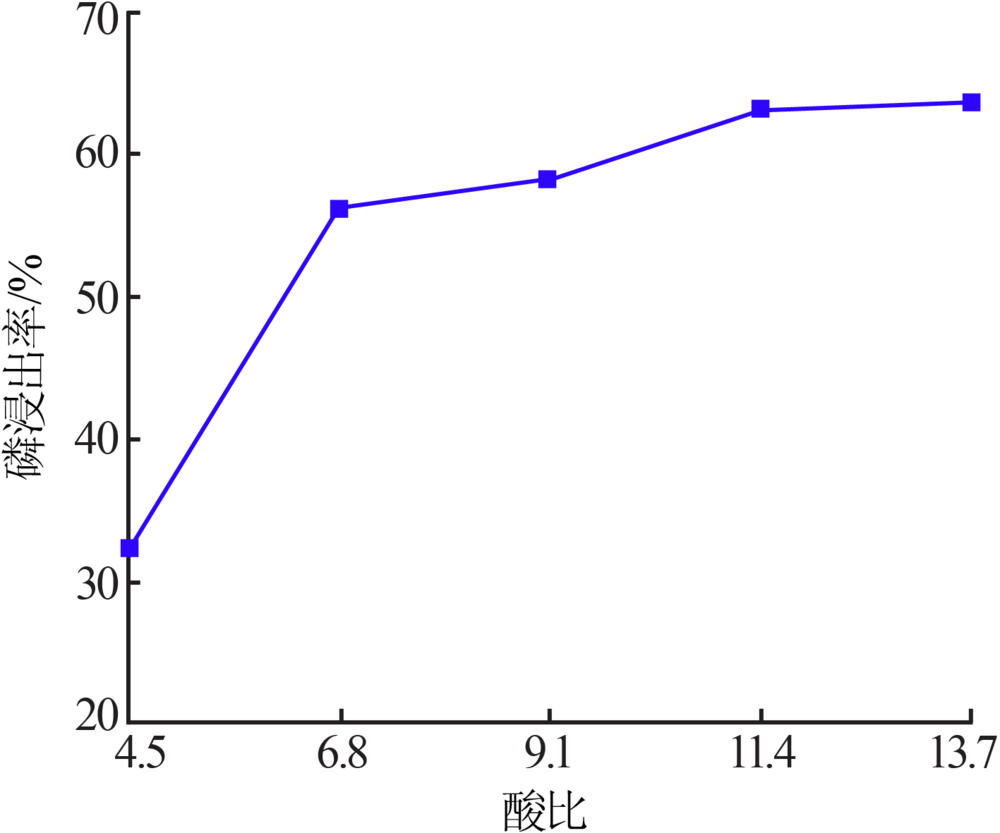
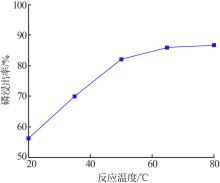
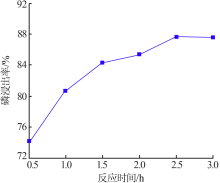
Research on influencing factors of industrial phosphoric acid decomposing phosphate rock and properties of by-products
Shao Yixin1,Zhang Zeqiang1( ),Huang Chaode2,Sun Hualin1
),Huang Chaode2,Sun Hualin1
- 1. School of Xingfa Mining Engineering,Wuhan Institute of Technology,Wuhan 430074,China
2. Xitieshan Branch of Western Mining Co.,Ltd.
-
Received:2019-01-22Online:2019-06-10Published:2020-05-12 -
Contact:Zhang Zeqiang E-mail:490062132@qq.com
CLC Number:
Cite this article
Shao Yixin,Zhang Zeqiang,Huang Chaode,Sun Hualin. Research on influencing factors of industrial phosphoric acid decomposing phosphate rock and properties of by-products[J]. Inorganic Chemicals Industry, 2019, 51(6): 49-52.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] | 傅忠德 . 浅谈湿法磷酸的生产方法[J]. 化学工程与装备, 2010(7):138-139. |
| [2] | 毛常明, 刘晶晶, 尹进华 , 等. 湿法磷酸的工艺研究进展[J]. 河北化工, 2005(4):14-16. |
| [3] | 邵一鑫, 张泽强, 孙桦林 , 等. 氢氧化钠分解磷石膏工艺研究[J]. 无机盐工业, 2017,49(9):52-54. |
| [4] | 李美 . 磷石膏品质的影响因素及其建材资源化研究[D]. 重庆:重庆大学, 2012. |
| [5] |
李相国, 陈嘉懿, 马保国 , 等. 粒度分布对磷石膏-石灰-粉煤灰胶结材物理性能的影响[J]. 化工学报, 2012,63(S1):230-234.
doi: 10.3969/j.issn.0438-1157.2012.z1.040 |
| [6] | 高洁, 赵国彦 . 磷石膏再结晶体力学特性试验研究[J]. 矿冶工程, 2013,33(2):18-21. |
| [7] | 阮耀阳, 罗惠华, 张泽强 , 等. 难选硅钙质胶磷矿正反浮选研究[J]. 矿冶工程, 2017,37(6):38-41. |
| [8] | 余静 . 利用低品位磷矿生产湿法磷酸的新工艺及动力学研究[D]. 成都:四川大学, 2005. |
| [9] | 刘代俊, 钟本和, 张允湘 . 磷酸复杂系统液固相反应动力学(Ⅱ)磷矿颗粒系统酸解动力学实验研究[J]. 化工学报, 2001,52(1):28-34. |
| [10] |
马保国, 茹晓红, 邹开波 , 等. 常压水热Ca-Na-Cl溶液中用磷石膏制备α-半水石膏[J]. 化工学报, 2013,64(7):2701-2707.
doi: 10.3969/j.issn.0438-1157.2013.07.053 |
| [11] | 高学顺, 陈江, 陈学玺 . 一种副产石膏晶须的湿法磷酸新工艺[J]. 无机盐工业, 2011,43(10):51-53. |
| [12] | 刘代俊, 钟本和 . 磷酸复杂系统液固相反应动力学(Ⅰ)分形介质中的扩散与反应机理[J]. 化工学报, 2000,61(5):620-625. |
| [13] | 吴佩芝 . 湿法磷酸[M]. 北京: 化学工业出版社, 1987. |
| [14] | 曾亚平, 党亚固, 费德君 . 制备高品质磷石膏的湿法磷酸新工艺的开发[J]. 化工进展, 2015,34(增刊1):167-172. |
| [15] | 彭家惠, 万体智, 汤玲 , 等. 磷石膏中杂质组成、形态、分布及其对性能的影响[J]. 中国建材科技, 2000(6):31-35. |
| [1] | Yin Wei,Huang Jin,Xie Tian,He Bingbing. Experimental study on high efficiency decomposition of phosphate rock by phosphoric acid [J]. Inorganic Chemicals Industry, 2020, 52(9): 34-36. |
| [2] | Wang Xiaoxiong,Zhang Jiangang,Zhang Wenjing,Chen Ye. Removal of calcium chloride from crude phosphoric acid by hydrochloric acid method [J]. Inorganic Chemicals Industry, 2020, 52(8): 17-19. |
| [3] | Wang Xiheng,Sun Wenzhe. Research progress on fluorine recovery technology in wet process phosphoric acid [J]. Inorganic Chemicals Industry, 2020, 52(8): 25-29. |
| [4] | Chen Yong,Yang Sanke,Xie Tian,Tao Shaocheng,Long Qinglan,Liu Xu. Research on preparation process of water-soluble ammonium polyphosphate with high polymerization rate [J]. Inorganic Chemicals Industry, 2020, 52(5): 50-52. |
| [5] | Wang Baoming,Lü Zhonghao,Li Huiyong,Liu Yong,Hua Quanxian,Tang Jianwei. Research progress on preparation of urea phosphate with wet-process phosphoric acid [J]. Inorganic Chemicals Industry, 2020, 52(11): 1-5. |
| [6] | LIU Fei, LI Tian-Xiang, JIE Tian, SHI Lian-Jun. Study on purification of purified wet-process phosphoric acid [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(2): 42-. |
| [7] | ZHANG Hai-Yan, MING Da-Zeng, JI Xiao-Ling, YANG Jin. Analysis on de-fluorination reaction principle of wet process phosphoric acid [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(1): 9-. |
| [8] | ZHOU Jing, BAI Ren-Liu, Zhou-Jun-Hong, WANG Jue, LUO Jun, JIA Shuang-Zhu. Preparation of high-quality potassium dihydrogen phosphate from wet-process phosphoric acid by extraction method [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(9): 21-. |
| [9] | WENG Yan-Ling, JIN Yang, LI Jun, JIA Xu-Hong, WU Hao-You. Study on demulsification of emulsification extraction of wet process phosphoric acid [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(5): 27-. |
| [10] | XIN Yang-Yang, YE Shi-Chao, LI Jun-Hong. Experimental extraction study of Kühni column with dispersed network [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(5): 42-. |
| [11] | YANG Wei-Hong, LI Xin-Zhu, HU Zhao-Ping. Summary for preparation of potassium dihydrogen phosphate from wet-process phosphoric acid [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(4): 7-. |
| [12] | ZOU Mi, MING Da-Zeng, LI Zhi-Xiang, YANG Jin, ZHANG Wen-Wen. Research progress in removing magnesium ions from wet-process phosphoric acid [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(2): 10-. |
| [13] | KUANG Guo-Ming. Discussion on process routes of wet-process phosphoric acid [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(4): 1-. |
| [14] | SUN Yi, LI Jun, ZHOU Kun, ZHANG Ya-Qing. Study on preparation of potassium dihydrogen phosphate from wet-process phosphoric acid by solvent extraction method [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(4): 34-. |
| [15] | KUANG Jia-Ling, WANG Yu- . Technology for preparation of potassium fluoborate from fluosilicic acid by-produced in wet-process phosphoric acid production [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(2): 39-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|