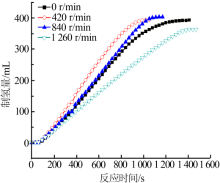
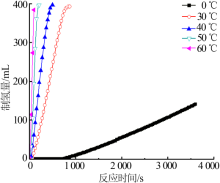
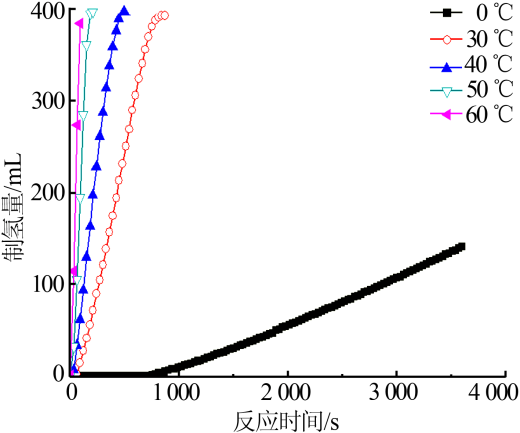
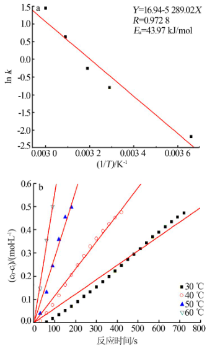
| [1] |
Brown H C, Brown C A . New,highly active metal catalysts for the hydrolysis of borohydride[J]. Journal of the American Chemical So-ciety, 1962,84(8):1493-1494.
|
| [2] |
孙海杰, 陈凌霞, 黄振旭 , 等. 第四周期过渡金属催化硼氢化钠分解制氢研究[J]. 无机盐工业, 2017,49(5):14-17.
|
| [3] |
徐东彦, 张华民, 叶威 . 硼氢化钠水解制氢[J]. 化学进展, 2007,19(10):1598-1605.
|
| [4] |
洪学斌, 王亚权 . NaBH4水解制氢技术[J]. 化学工业与工程, 2005,22(6):467-471.
|
| [5] |
曲健林, 韩敏, 张秀丽 , 等. 棉杆活性炭负载Co-B催化剂催化硼氢化钠水解制氢的性能[J]. 化工学报, 2015,66(1):105-113.
doi: 10.11949/j.issn.0438-1157.20140645
|
| [6] |
蔡凡, 沈晓晨, 戴敏 , 等. CoB/C催化硼氢化钠水解制氢的性能[J]. 无机化学学报, 2013,29(4):689-696.
doi: 10.3969/j.issn.1001-4861.2013.00.143
|
| [7] |
Dong H, Yang H, Ai X , et al. Hydrogen production from catalytic hydrolysis of sodium borohydride solution using nickel boride cat-alyst[J]. International Journal of Hydrogen Energy, 2003,28(10):1095-1100.
doi: 10.1016/S0360-3199(02)00235-5
|
| [8] |
Tian H, Guo Q, Xu D . Hydrogen generation from catalytic hydrolysis of alkaline sodium borohydride solution using attapulgite clay-sup-ported Co-B catalyst[J]. Journal of Power Sources, 2010,195(8):2136-2142.
doi: 10.1016/j.jpowsour.2009.10.006
|
| [9] |
苗强, 黄子健, 李其名 , 等. 硼化钴/石墨烯非晶合金催化剂制备及性能研究[J]. 无机盐工业, 2018,50(7):80-83.
|
| [10] |
于晓飞, 鲍新侠, 李其名 , 等. CoB/Al2O3非晶态合金催化剂的制备及在催化制氢中的应用[J]. 无机盐工业, 2015,47(1):59-62.
|
| [11] |
Wang Y, Lu Y, Wang D , et al. Hydrogen generation from hydrolysis of sodium borohydride using nanostructured Ni-B catalysts[J]. In-ternational Journal of Hydrogen Energy, 2016,41(36):16077-16086.
|
| [12] |
李赛, 王丽娜 . Mo掺杂的Ni-B非晶态合金的制备及催化硼氢化钠水解制氢[J]. 山东化工, 2016,45(12):4-6.
|
| [13] |
赵士夺, 李芳, 李其明 , 等. 分子筛USY负载硼化钴非晶态合金催化剂的制备及其在催化硼氢化钠水解制氢中的应用[J]. 应用化学, 2016,33(6):655-660.
|
| [14] |
梁志花, 李其明, 李芳 , 等. ZIF-7负载CoB催化剂的制备及其在制氢中的应用[J]. 应用化工, 2016,45(10):1832-1835.
|
| [15] |
梁志花, 李其明, 李芳 , 等. 棒状丝光沸石负载CoB催化剂的制备及其应用[J]. 化工新型材料, 2017,45(2):88-90.
|