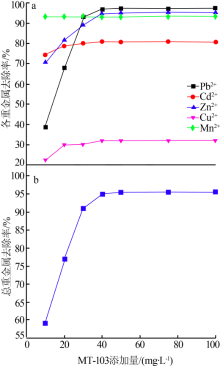
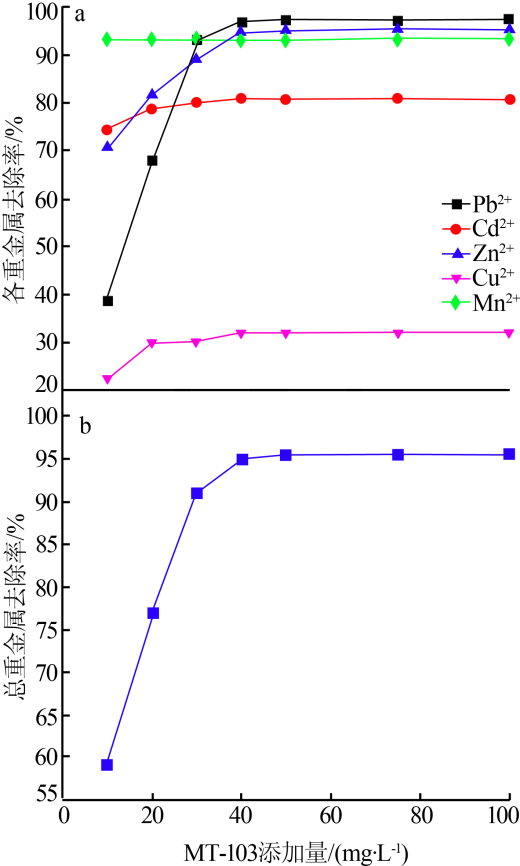
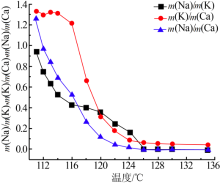
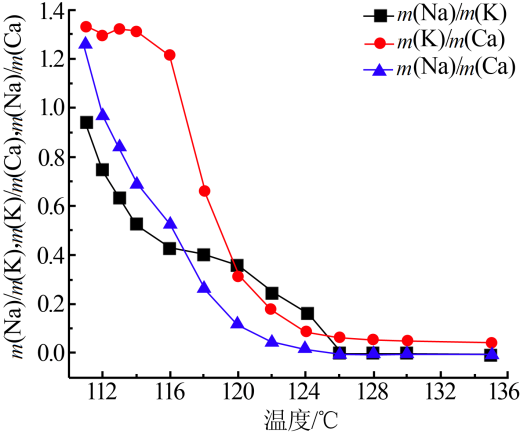
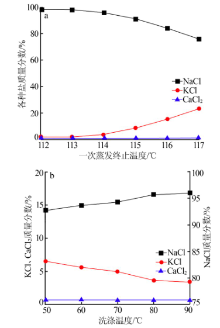
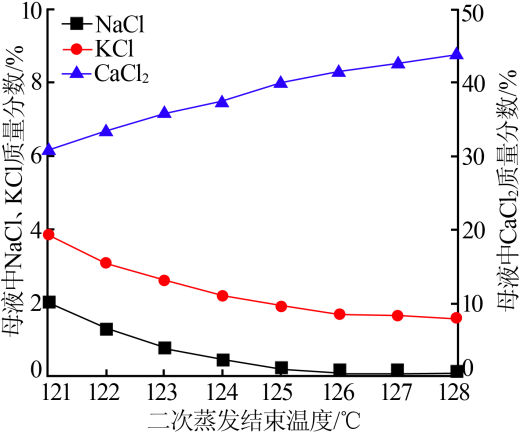
| [1] |
蒋旭光, 常威 . 生活垃圾焚烧飞灰的处置及应用概况[J]. 浙江工业大学学报, 2015,43(1):7-11.
|
| [2] |
Bobirică C, Shim J H, Park J Y . Leaching behavior of fly ash-waste glass and fly ash-slag-waste glass-based geopolymers[J]. Geramics International, 2018,44(6):5886-5893.
|
| [3] |
靳美娟 . 城市生活垃圾焚烧飞灰水泥固化技术研究[J]. 环境工程学报, 2016,10(6):3235-3241.
|
| [4] |
常威 . 生活垃圾焚烧飞灰的水洗及资源化研究[D]. 浙江:浙江大学, 2016.
|
| [5] |
Zhan G, Guo Z C . Water leaching kinetics and recovery of potassium salt from sintering dust[J]. Transactions of Nonferrous Metals So-Society of China, 2013,23(12):3770-3779.
|
| [6] |
Zhan G, Guo Z C . Basic properties of sintering dust from iron and steel plant and potassium recovery[J]. Journal of Environmental Sci-ences, 2013,25(6):1226-1234.
doi: 10.1016/s1001-0742(12)60168-5
pmid: 24191613
|
| [7] |
张更宇, 张冬冬 . 化学沉淀法处理电镀废液中重金属的实验研究[J]. 山东化工, 2016,45(16):215-220.
|
| [8] |
Kenawy I M, Hafez M A H, Ismail M A , et al.Adsorption of Cu(Ⅱ),Cd(Ⅱ),Hg(Ⅱ),Pb(Ⅱ) and Zn(Ⅱ) from aqueous single metal solutions by guanyl-modified cellulose[J]. International Journal of Biological Macromolecules, 2018,107:1538-1549.
doi: 10.1016/j.ijbiomac.2017.10.017
pmid: 28988841
|
| [9] |
李学峰, 朱蕾, 高焕新 , 等. 不同沸石吸附铅离子的对比研究[J]. 无机盐工业, 2011,43(8):21-24.
|
| [10] |
胡栋梁, 方亚平, 温会涛 , 等. 电渗析和反渗透耦合深度处理制革高盐废水的研究[J]. 水处理技术, 2017,43(11):107-111.
|
| [11] |
Suthanthararajan R, Ravindranath E, Chits K , et al. Membrane app-lication for recovery and reuse of water from treated tannery waste-water[J]. Desalination, 2004,164(2):151-156.
doi: 10.1016/S0011-9164(04)00174-2
|
| [12] |
雷兆武, 孙颖 . 离子交换技术在重金属废水处理中的应用[J]. 环境科学与管理, 2008,33(10):82-84.
|
| [13] |
Ya V, Martin N, Chou Y H , et al. Electrochemical treatment for si-multaneous removal of heavy metals and organics from surface fi-nishing wastewater using sacrificial iron anode[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018,83:107-114.
|
| [14] |
凌永生, 金宜英, 聂永丰 . 焚烧飞灰水泥窑煅烧资源化水洗预处理实验研究[J]. 环境保护科学, 2012,38(4):1-5.
|
| [15] |
白晶晶, 闫大海, 李丽 , 等. CO2去除垃圾焚烧飞灰水洗液中Pb和Zn的工艺条件[J]. 环境科学研究, 2012,25(7):809-814.
|
| [16] |
Mangialardi T . Disposal of MSWI fly ash through a combined wash-ing-immobilisation process[J]. Journal of Hazardous Materials, 2003,98:225-240.
doi: 10.1016/s0304-3894(02)00359-x
pmid: 12628790
|
| [17] |
Djedidi Z, Bouda M, Souissi M A , et al. Metals removal from soil,flyash and sewage sludge leachates by precipitation and dewatering properties of the generated sludge[J]. Journal of Hazardous Mate-rials, 2009,172:1372-1382.
doi: 10.1016/j.jhazmat.2009.07.144
pmid: 19713039
|
| [18] |
王彦飞, 杨静, 王婧莹 , 等. 煤化工高浓盐废水蒸发处理工艺进展[J]. 无机盐工业, 2017,49(1):10-14.
|
| [19] |
韩大健, 王文祥, 孙水裕 , 等. 城市生活垃圾焚烧飞灰中钾盐浸出研究[J]. 环境科学学报, 2017,37(6):2223-2231.
|
| [20] |
张晓樵 . 生活垃圾焚烧飞灰毒性浸出规律及水洗预处理废水资源化处理探索[D]. 上海:上海大学, 2015.
|
 )
)