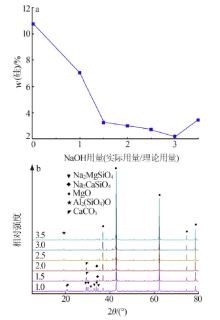
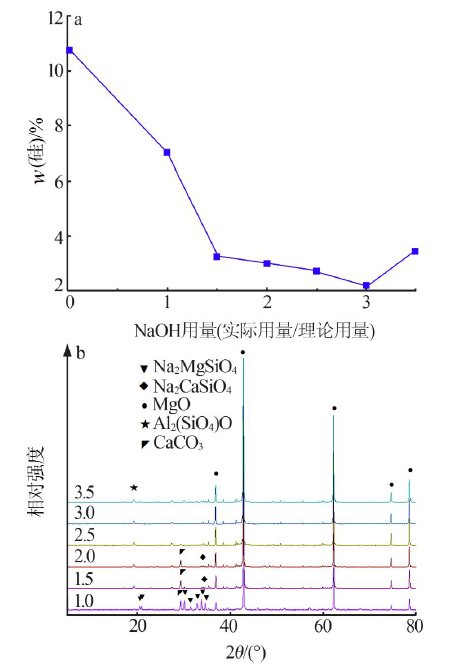
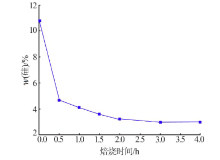
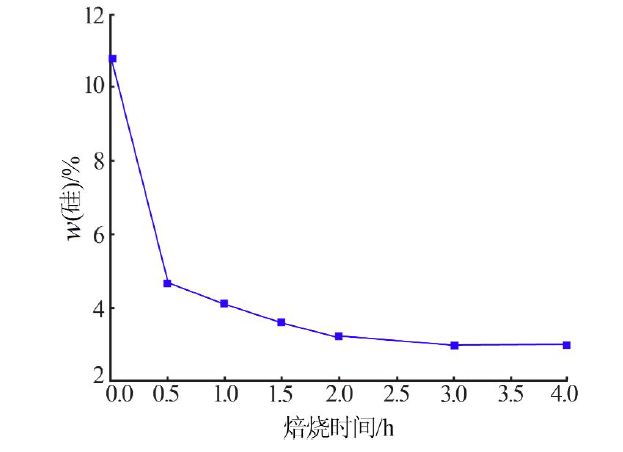
| [1] |
秦雅静, 朱德山. 我国水镁石矿资源利用现状及展望[J]. 中国非金属矿工业导刊, 2014(6):1-3.
|
| [2] |
符剑刚, 王晖, 陈立, 等. 从镍蛇纹石矿石中浸出Ni,Mg的试验研究[J]. 湿法冶金, 2008(2):92-95.
|
| [3] |
宋鹏程, 彭同江, 孙红娟, 等. 纤蛇纹石石棉尾矿综合利用新进展[J]. 中国非金属矿工业导刊, 2016(2):14-17,23.
|
| [4] |
宋贝, 刘超, 郑水林, 等. 用蛇纹石硫酸铵焙烧法制备超细氢氧化镁的研究[J]. 非金属矿, 2013,36(3):63-65.
|
| [5] |
宋鹏程, 彭同江, 孙红娟, 等. 纤蛇纹石焙烧去金属氧化物制备纤维状二氧化硅[J]. 硅酸盐学报, 2015,43(5):692-698.
|
| [6] |
余玉操, 彭同江, 孙红娟, 等. 温石棉尾矿酸法焙烧产物提取氧化镁的研究[J]. 非金属矿, 2016,39(2):1-4.
|
| [7] |
李学军. 天然蛇纹石活性机理初探[C]// 中国地质学会.第二届全国环境矿物学学术研讨会论文集, 2004: 67-71.
|
| [8] |
Teixeira A P C, Santos E M, Vieira A F P, et al. Use of chrysotile to produce highly dispersed K-doped MgO catalyst for biodiesel synjournal[J]. Chemical Engineering Journal, 2013,232:104-110.
|
| [9] |
Ballotin F C, Cibaka Thérèse Ebambi, Ribeiro-Santos T A, et al. K2MgSiO4:A novel K+-trapped biodiesel heterogeneous catalyst produced from serpentinite Mg3Si2O5(OH)4[J]. Journal of Molecular Catalysis A:Chemical, 2016,422:258-265.
|
| [10] |
李世青, 巩媛媛, 赵文娟. 蛇纹石石棉煅烧改性研究[J]. 矿产综合利用, 2008(3):25-27.
|
| [11] |
徐敏. 碱分解含镁硅酸盐的光谱学研究[D]. 沈阳:东北大学, 2014.
|
| [12] |
Wu X F, Hu G S, Wang B B, et al. Synjournal and characterization of superfine magnesium hydroxide with monodispersity[J]. Journal of Crystal Growth, 2008,310(2):457-461.
|
| [13] |
秦宏宇, 刘瑞. 吉林通化集安蛇纹石质玉的矿物成分与成因分析[J]. 岩石矿物学杂志, 2016,35(2):344-348.
|
| [14] |
谢晶曦, 常俊标, 王绪明. 红外光谱在有机化学和药物化学中的应用[M]. 北京: 科学出版社, 2001.
|
| [15] |
Roda M, Paluszkiewicz C. The structural role of alkaline earth ions in oxyfluoride aluminosilicate glasses—Infrared spectroscopy study[J]. Vibrational Spectroscopy, 2008,48(2):246-250.
|
 ),Zhong Jianchu1,2(
),Zhong Jianchu1,2( ),Wang Xiaotian1,2
),Wang Xiaotian1,2